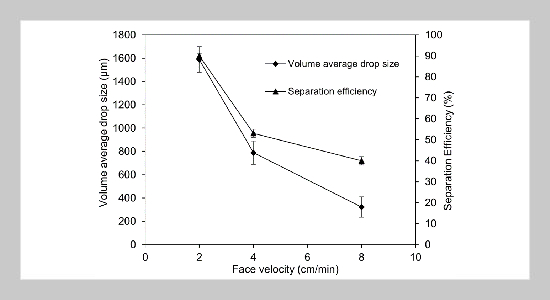
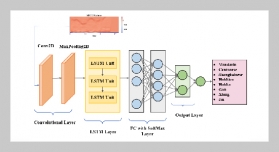
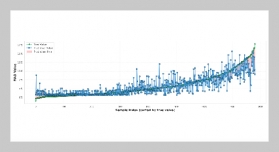
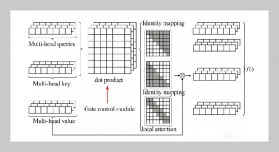
- [1] W. Stone, G. Bessee, and C. Stanfel, (2009) “Diesel Fuel/Water Separation Test Methods—Where We Are and Where We Are Going" SAE Int J Fuels Lubr 2(1): 317–323. DOI: 10.4271/2009-01-0875.
- [2] C. Stanfel, (2009) “Fuel filtration: Protecting the diesel engine" Filtration & Separation 46(3): 22–25. DOI: 10.1016/S0015-1882(09)70124-7.
- [3] M. Nadeem, C. Rangkuti, K. Anuar, M. R. U. Haq, I. B. Tan, and S. S. Shah, (2006) “Diesel engine performance and emission evaluation using emulsified fuels stabilized by conventional and gemini surfactants" Fuel 85(14–15): 2111–2119. DOI: 10.1016/j.fuel.2006.03.013.
- [4] T. Kadota and H. Yamasaki, (2002) “Recent advances in the combustion of water fuel emulsion" Prog Energy Combust Sci 28(5): 385–404. DOI: 10.1016/S0360- 1285(02)00005-9.
- [5] A. M. Ithnin, H. Noge, H. A. Kadir, and W. Jazair, (2014) “An overview of utilizing water-in-diesel emulsion fuel in diesel engine and its potential research study" Journal of the Energy Institute 87(4): 273–288. DOI: 10.1016/J.JOEI.2014.04.002.
- [6] M. Hosseini, (2016) “Coalescence behaviour of water droplets in water-oil interface under pulsatile electric fields" Chin J Chem Eng 24(9): 1147–1153. DOI: 10.1016/J.CJCHE.2016.04.007.
- [7] P. S. Kulkarni, S. U. Patel, and G. G. Chase, (2012) “Layered hydrophilic/hydrophobic fiber media for water-in�oil coalescence" Sep Purif Technol 85: 157–164. DOI: 10.1016/j.seppur.2011.10.004.
- [8] O. J. Ajogbeje, A. Stammitti-Scarpone, S. Cao, T. Akanni, S. Ng, and E. J. Acosta, (2021) “Separation of Emulsions with Fibrous Filter-Coalescers" Langmuir 37(51): DOI: 10.1021/ACS.LANGMUIR.1C00333.
- [9] R. C. Brown, (1995) “Air filtration: an integrated ap�proach to the theory and applications of fibrous filters" J Aerosol Sci 26(1): 171.
- [10] S. S. Sareen, P. M. Rose, R. C. Gudesen, and R. C. Kint�ner, (1966) “Coalescence in fibrous beds" AIChE Journal 12(6): 1045–1050. DOI: 10.1002/AIC.690120603.
- [11] N. N. Zaki, R. G. Carbonell, and P. K. Kilpatrick, (2003) “A Novel Process for Demulsification of Water-in�Crude Oil Emulsions by Dense Carbon Dioxide" Ind Eng Chem Res 42(25): 6661–6672. DOI: 10.1021/IE0303597.
- [12] S. Bansal, V. von Arnim, T. Stegmaier, and H. Planck, (2011) “Effect of fibrous filter properties on the oil-in�water-emulsion separation and filtration performance" J Hazard Mater 190(1–3): 45–50. DOI: 10.1016/J.JHAZMAT.2011.01.134.
- [13] D. Thomas, P. Penicot, P. Contal, D. Leclerc, and J. Vendel, (2001) “Clogging of fibrous filters by solid aerosol particles Experimental and modelling study" Chem Eng Sci 56(11): 3549–3561. DOI: 10.1016/S0009-2509(01)00041-0.
- [14] A. D. Gadhave and G. G. Chase, (2021) “Coalescence of emulsified water drops in ULSD using a steel mesh electrowet coalescer" Sep Purif Technol 254: 117675. DOI: 10.1016/J.SEPPUR.2020.117675.
- [15] V. G. Levich. Physicochemical hydrodynamics. Engle�wood Cliffs, N.J: Prentice-Hall, 1962.
- [16] S. Mhatre, V. Vivacqua, M. Ghadiri, A. M. Abdullah, M. J. Al-Marri, A. Hassanpour, B. Hewakandamby, B. Azzopardi, and B. Kermani, (2015) “Electrostatic phase separation: A review" Chemical Engineering Research and Design 96: 177–195. DOI: 10.1016/J.CHERD.2015.02.012.
- [17] Y. Li, L. Cao, D. Hu, and C. Yang, (2017) “Uncommon wetting on a special coating and its relevance to coales�cence separation of emulsified water from diesel fuel" Sep Purif Technol 176: 313–322. DOI: 10.1016/J.SEPPUR.2016.11.058.
- [18] H. Gong, W. Li, X. Zhang, Y. Peng, B. Yu, and Y. Mou, (2020) “Simulation of the coalescence and breakup of water-in-oil emulsion in a separation device strengthened by coupling electric and swirling centrifugal fields" Sep Purif Technol 238: 116397. DOI: 10.1016/J.SEPPUR.2019.116397.
- [19] I. G. Harpur, N. J. Wayth, A. G. Bailey, M. T. Thew, T. J. Williams, and O. Urdahl, (1997) “Destabilisation of water-in-oil emulsions under the influence of an A.C. electric field: Experimental assessment of performance" J Electrostat 40–41: 135–140. DOI: 10.1016/S0304-3886(97)00027-2.
- [20] J. S. Eow and M. Ghadiri, (2002) “Electrostatic enhance�ment of coalescence of water droplets in oil: a review of the technology" Chemical Engineering Journal 85(2–3): 357–368. DOI: 10.1016/S1385-8947(01)00250-9.
- [21] T. Hirato, K. Koyama, T. Tanaka, Y. Awakura, and H. Majima, (1991) “Demulsification of Water-in-Oil Emul�sion by an Electrostatic Coalescence Method" Materi�als Transactions, JIM 32(3): 257–263. DOI: 10.2320/MATERTRANS1989.32.257.
- [22] D. Yang, Y. Sun, M. Ghadiri, H. Wu, H. Qiao, L. He, X. Luo, and Y. Lü, (2019) “Effect of hydrolyzed polyacrylamide used in polymer flooding on droplet– interface electro-coalescence: Variation of critical electric field strength of partial coalescence" Sep Purif Technol 227: 115737. DOI: 10.1016/J.SEPPUR.2019.115737.
- [23] J. S. Eow, M. Ghadiri, A. O. Sharif, and T. J. Williams, (2001) “Electrostatic enhancement of coalescence of water droplets in oil: a review of the current understanding" Chemical Engineering Journal 84(3): 173–192. DOI: 10.1016/S1385-8947(00)00386-7.
- [24] P. J. Bailes and S. K. L. Larkai, (1984) “Influence of phase ration on electrostatic coalescence of water-in-oil dispersions" CEGB Res.; (United Kingdom) 62(1):
- [25] M. M. Mohammadi, S. Shahhosseini, and M. Bayat, (2014) “Electrocoalescence of binary water droplets falling in oil: Experimental study" Chemical Engineering Re�search and Design 92(11): 2694–2704. DOI: 10.1016/J.CHERD.2014.01.019.
- [26] H. Aryafar and H. P. Kavehpour, (2009) “Electrocoales�cence: Effects of DC electric fields on coalescence of drops at planar interfaces" Langmuir 25(21): 12460–12465. DOI: 10.1021/LA902758U.
- [27] H. B. Hauertmann, W. Degener, and K. Schogerl, (1989) “Electrostatic Coalescence: Reactor, Process Con�trol, and Important Parameters" Sep Sci Technol 24(3– 4): 253–273. DOI: 10.1080/01496398908049766.
- [28] A. T. Yasir, A. H. Hawari, M. Talhami, M. Baune, J. Thöming, and F. Du, (2023) “The impact of electric field on the demulsification efficiency in an electro-coalescence process" J Electrostat 122: 103796. DOI: 10.1016/J.ELSTAT.2023.103796.
- [29] S. Less and R. Vilagines, (2012) “The electrocoalescers’ technology: Advances, strengths and limitations for crude oil separation" J Pet Sci Eng 81: 57–63. DOI: 10.1016/J.PETROL.2011.12.003.
- [30] S. Mhatre and R. Thaokar, (2015) “Electrocoalescence in non-uniform electric fields: An experimental study" Chemical Engineering and Processing: Process In�tensification 96: 28–38. DOI: 10.1016/J.CEP.2015.07.025.
- [31] F. Mugele and J.-C. Baret, (2005) “Electrowetting: from basics to applications" J. Phys.: Condens. Matter 17: 705–774. DOI: 10.1088/0953-8984/17/28/R01.
- [32] A. S. Aljuhani and G. G. Chase, (2016) “Electrowetting water droplet contact angle relaxation on coated stain�less steel plates" International Journal of Surface Sci�ence and Engineering 10(3): 224–239. DOI: 10.1504/ IJSURFSE.2016.076995.
- [33] C. Quilliet and B. Berge, (2001) “Electrowetting: a re�cent outbreak" Curr Opin Colloid Interface Sci 6(1): 34–39. DOI: 10.1016/S1359-0294(00)00085-6.
- [34] A. Klingner and F. Mugele, (2004) “Electrowetting�induced morphological transitions of fluid microstruc�tures" J Appl Phys 95(5): 2918–2920. DOI: 10.1063/1.1643771.
- [35] A. Kumar, I. Ahmad, and M. Pathak, (2023) “Droplet impact on a hydrophobic surface integrated with elec�trowetting technique" Colloids Surf A Physicochem Eng Asp 656: 130423. DOI: 10.1016/J.COLSURFA.2022.130423.
- [36] G. Chase and A. Bandekar, (2016) “Coalescence of Wa�ter Drops in Water-ULSD Dispersions via Electrowet�ting" Journal of Coating Science and Technology 3(1): 41–49. DOI: 10.6000/2369-3355.2016.03.01.5.
- [37] I. F. Guha and K. K. Varanasi, (2019) “Low-Voltage Surface Electrocoalescence Enabled by High-K Dielectrics and Surfactant Bilayers for Oil–Water Separation" ACS Applied Materials & Interfaces 11(38): 34812–34818. DOI: 10.1021/acsami.9b01477.
- [38] J. Zhou, A. Bandekar, and G. G. Chase, (2018) “Eval�uation of electrowet coalescer in series with PVDF-HFP electrospun fiber membranes for separation of water from ULSD" Fuel 225: 111–117. DOI: 10.1016/J.FUEL.2018.03.142.
- [39] É. Ruiz-Gutiérrez and R. Ledesma-Aguilar, (2019) “Lattice-Boltzmann Simulations of Electrowetting Phenom�ena" Langmuir 35(14): 4849–4859. DOI: 10.1021/ACS.LANGMUIR.9B00098.
- [40] S. Fu, G. Deng, H. Dong, Y. Sun, Y. Hu, F. Zhou, and H. Yaun, (2023) “Numerical simulation of oil dewatering in a disc centrifuge based on PBM model" Exp Comput Multiph Flow 5(2): 212–220. DOI: 10.1007/S42757- 022-0137-7.
- [41] N. Ayuba, R. de Borba Buhler, L. S. da Silva, and T. J. Lopes, (2019) “Application of density-viscosity in predict�ing oil-water flow profile in horizontal pipe" Petroleum 5(2): 155–162. DOI: 10.1016/J.PETLM.2018.12.001.
- [42] Z. Qi, Z. Sun, N. Li, Q. Chen, W. Liu, W. Li, and J. Sun, (2023) “Electrophoretic coalescence behavior of oil droplets in oil-in-water emulsions containing SDS under DC electric field: A molecular dynamics study" Fuel 338: 127258. DOI: 10.1016/j.fuel.2022.127258.
- [43] X. Luo, H. Yin, J. Ren, H. Yan, Y. Lü, and L. He, (2019) “Electrocoalescence criterion of conducting droplets sus�pended in a viscous fluid" The Journal of Physical Chemistry C 123(32): 19588–19595. DOI: 10.1021/acs.jpcc.9b04357.
- [44] Y. Chen, S. Narayan, and C. S. Dutcher, (2020) “Phase�dependent surfactant transport on the microscale: Interfa�cial tension and droplet coalescence" Langmuir 36(49): 14904–14923. DOI: 10.1021/acs.langmuir.0c02476.
- [45] C. Lesaint, W. R. Glomm, L. E. Lundgaard, and J. Sjöblom, (2009) “Dehydration efficiency of AC electrical fields on water-in-model-oil emulsions" Colloids Surf A Physicochem Eng Asp 352(1–3): 63–69. DOI: 10.1016/J.COLSURFA.2009.09.051.
- [46] T. J. Williams and A. G. Bailey, (1986) “Changes in the size distribution of a water-in-oil emulsion due to electric field induced coalescence" IEEE Transactions on industry applications (3): 536–541. DOI: 10.1109/TIA.1986.4504755.