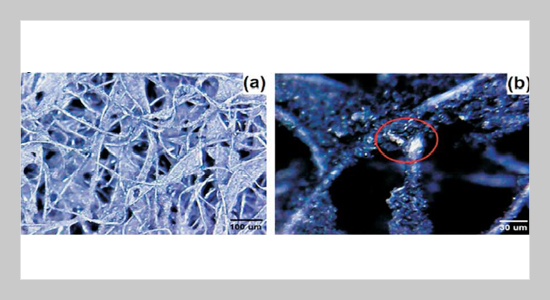
REFERENCES
- [1] Maddah, H., and A. Chogle (2016) Biofouling in reverse osmosis: phenomena, monitoring, controlling and remediation, Appl. Water Sci. 7(6), 2637–2651.
- [2] Maddah, H. A., et al. (2017) Determination of the treatment efficiency of different commercial membrane modules for the treatment of groundwater, J. Mater. Environ. Sci. 8(6), 2006–2012.
- [3] Maddah, H. A., and A. S. Alzhrani (2017) Quality monitoring of various local and imported brands of bottled drinking water in Saudi Arabia, World J. Eng. Technol. 5(4), 551–563. doi: 10.4236/wjet.2017.54047
- [4] Maddah, H. A., and A. M. Chogle (2015) Applicability of low pressure membranes for wastewater treatment with cost study analyses, Membr. Water Treat. 6(6), 477–488. doi: 10.12989/mwt.2015.6.6.477
- [5] Strathmann H, D. E., and L. Giorno (2006) An Introduction to Membrane Science and Technology, Roma: CNR.
- [6] Fane, A. G. (1996) Membranes for water production and wastewater reuse, Desalination 106(1–3), 1–9. doi: 10.1016/0011-9164(96)00085-9
- [7] Qureshi, B. A., S. M. Zubair, A. K. Sheikh, A. Bhujle, and S. Dubowsky (2013) Design and performance evaluation of reverse osmosis desalination systems: an emphasis on fouling modeling, Appl. Therm. Eng. doi: 10.1016/j.applthermaleng.2013.06.058
- [8] Van der Bruggen, B., and C. Vandecasteele (2002) Distillation vs. membrane filtration: overview of process evolutions in seawater desalination, Desalination. doi: 10.1016/S0011-9164(02)00259-X
- [9] Yurekli, Y. (2016) Removal of heavy metals in wastewater by using zeolite nano-particles impregnated polysulfone membranes, J. Hazard. Mater. doi: 10.1016/ j.jhazmat.2016.01.064
- [10] Maddah, M., and H. A. Almughwi (2017) Application of the solution-diffusion model to optimize water flux in reverse osmosis desalination plants, AWWA/AMTA Membrane Technology Conference and Exposition.
- [11] Hegazi, H. A. (2013) Removal of heavy metals from wastewater using agricultural and industrial wastes as adsorbents, HBRC J. doi: 10.1016/j.hbrcj.2013.08.004
- [12] Karnib, M., A. Kabbani, H. Holail, and Z. Olama (2014) Heavy metals removal using activated carbon, silica and silica activated carbon composite, Energy Procedia. doi: 10.1016/j.egypro.2014.06.014
- [13] Fil, B. A., M. T. Yilmaz, S. Bayar, and M. T. Elkoca (2014) Investigation of adsorption of the dyestuff astrazon red violet 3rn (basic violet 16) on montmorillonite clay, Brazilian J. Chem. Eng. doi: 10.1590/ S0104-66322014000100016
- [14] Lemley A, K. B., and L. Wagenet (1995) Activated Carbon Treatment of Drinking Water, New York.
- [15] Mohamad Said, K. A., et al. (2017) Effect of activated carbon in polysufone-polyethyleneimine-silver composite membrane towards adsorption of chromium (Cr), lead (Pb), silver (Ag) and cadmium (Cd) in synthetic wastewater, J. Mater. Environ. Sci.
- [16] Freundlich, H. M. F. (1906) Adsorption in solution, Z. Phys. Chem.
- [17] Lykins Jr BW, C. R., E. E. Geldreich, J. Q. Adams, and J. C. Ireland (1984) Granular activated carbon for removing nontrihalomethane organics from drinking water.
- [18] Lalezary S, M. M., and M. Pirbazari (1986) Evaluating activated carbons for removing low concentrations of taste-and odor-producing organice, Am. Water Work. Assoc. 76–82. doi: 10.1002/j.1551-8833.1986. tb05851.x
- [19] Wagenet L, S. M., and K. Mancl (1995) Home water treatment, Natural Resource, Agriculture, and Engineering Service (NRAES).
- [20] Voudrias, E. A., R. A. Larson, and V. L. Snoeyink (1985) Effects of activated carbon on the reactions of combined chlorine with phenols, Water Res. doi: 10. 1016/0043-1354(85)90150-2
- [21] Maddah, H. A., and M. A. Shihon (2018) Activated carbon cloth for desalination of brackish water using capacitive deionization, Desalination and Water Treatment.
- [22] Laxman, K., M. T. Z. Myint, R. Khan, T. Pervez, and J. Dutta (2015) Effect of a semiconductor dielectric coating on the salt adsorption capacity of a porous electrode in a capacitive deionization cell, Electrochim. Acta 166, 329–337. doi: 10.1016/j.electacta.2015.03. 049
- [23] Myint, M. T. Z., and J. Dutta (2012) Fabrication of zinc oxide nanorods modified activated carbon cloth electrode for desalination of brackish water using capacitive deionization approach, Desalination 305, 24–30. doi: 10.1016/j.desal.2012.08.010
- [24] Ryoo, M. W., J. H. Kim, and G. Seo (2003) Role of titania incorporated on activated carbon cloth for capacitive deionization of NaCl solution, J. Colloid Interface Sci. 264(2), 414–419. doi: 10.1016/S0021-9797(03) 00375-8
- [25] Myint, M. T. Z., S. H. Al-Harthi, and J. Dutta (2014) Brackish water desalination by capacitive deionization using zinc oxide micro/nanostructures grafted on activated carbon cloth electrodes, Desalination 344, 236– 242. doi: 10.1016/j.desal.2014.03.037
- [26] Chang, L. M., X. Y. Duan, and W. Liu (2011) Preparation and electrosorption desalination performance of activated carbon electrode with titania, Desalination270(1–3), 285–290. doi: 10.1016/j.desal.2011.01.008
- [27] Langton, N. H., and D. Matthews (1958) The dielectric constant of zinc oxide over a range of frequencies, Br. J. Appl. Phys. doi: 10.1088/0508-3443/9/11/308
- [28] Wypych, A., et al. (2014) Dielectric properties and characterisation of titanium dioxide obtained by different chemistry methods, J. Nanomater. doi: 10.1155/ 2014/124814
- [29] Shkal, F., S. G. Lopez, D. Slocombe, and A. Porch (2018) Microwave characterization of activated carbons, J. Comput. Commun. doi: 10.4236/jcc.2018. 61012
- [30] Behzadi, G., and H. Golnabi (2010) Investigation of conductivity effects on capacitance measurements of water liquids using a cylindrical capacitive sensor, J. Appl. Sci. doi: 10.3923/jas.2010.261.268
- [31] Lee, T., Z. A. Zubir, F. M. Jamil, A. Matsumoto, and F. Y. Yeoh (2014) Combustion and pyrolysis of activated carbon fibre from oil palm empty fruit bunch fibre assisted through chemical activation with acid treatment, J. Anal. Appl. Pyrolysis. doi: 10.1016/j.jaap.2014. 10.010
- [32] Porada, S., R. Zhao, A. Van Der Wal, V. Presser, and P. M. Biesheuvel (2013) Review on the science and technology of water desalination by capacitive deionization, Progress in Materials Science 58(8), 1388–1442. doi: 10.1016/j.pmatsci.2013.03.005
- [33] Suss, M. E., S. Porada, X. Sun, P. M. Biesheuvel, J. Yoon, and V. Presser (2015) Water desalination via capacitive deionization: what is it and what can we expect from it? Energy Environ. Sci. 8(8), 2296–2319. doi: 10.1039/C5EE00519A
- [34] Waddington, T. C. (1966) Ionic radii and the method of the undetermined parameter, Trans. Faraday Soc. doi: 10.1039/tf9666201482
- [35] Ma, X., P. Chen, M. Zhou, Z. Zhong, F. Zhang, and W. Xing (2017) Tight ultrafiltration ceramic membrane for separation of dyes and mixed salts (both NaCl/ Na2SO4) in textile wastewater treatment, Ind. Eng. Chem. Res. doi: 10.1021/acs.iecr.7b01440
- [36] Fatehizadeh, A., E. Taheri, M. M. Amin, M. Mahdavi, and N. Moradi (2018) Sodium and potassium removal from brackish water by nanofiltration membrane: single and binary salt mixtures, Desalin. Water Treat. doi: 10.5004/dwt.2018.21900
- [37] Krieg, H. M., S. J. Modise, K. Keizer, and H. W. J. P. Neomagus (2005) Salt rejection in nanofiltration for single and binary salt mixtures in view of sulphate removal, Desalination. doi: 10.1016/j.desal.2004.05. 005