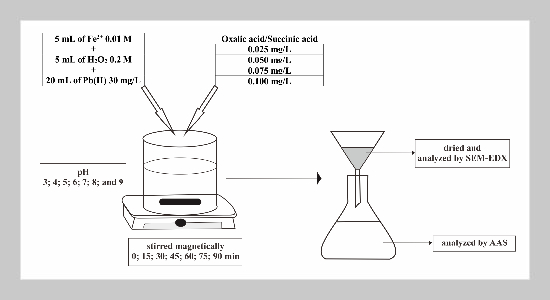
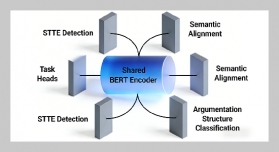
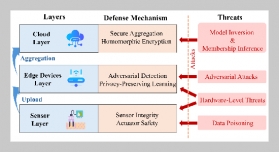
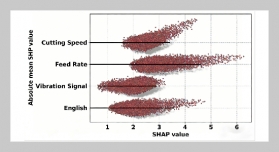
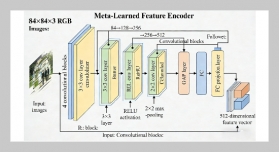
- [1] O. B. Akpor, (2014) “Heavy Metal Pollutants in Wastew�ater Effluents: Sources, Effects and Remediation" Ad�vances in Bioscience and Bioengineering 2(4): 37. DOI: 10.11648/j.abb.20140204.11.
- [2] A. Kumar, A. Kumar, M. Cabral-Pinto, A. K. Chaturvedi, A. A. Shabnam, G. Subrahmanyam, R. Mondal, D. K. Gupta, S. K. Malyan, S. S. Kumar, S. A. Khan, and K. K. Yadav, (2020) “Lead toxicity: Health hazards, influence on food Chain, and sustainable remediation approaches" International Journal of En�vironmental Research and Public Health 17(7): DOI: 10.3390/ijerph17072179.
- [3] M. E. Argun and S. Dursun, (2008) “A new approach to modification of natural adsorbent for heavy metal ad�sorption" Bioresource Technology 99(7): 2516–2527. DOI: 10.1016/j.biortech.2007.04.037.
- [4] A. L. Wani, A. Ara, and J. A. Usmani, (2015) “Lead toxicity: A review" Interdisciplinary Toxicology 8(2): 55–64. DOI: 10.1515/intox-2015-0009.
- [5] A. E. Charkiewicz and J. R. Backstrand, (2020) “Lead toxicity and pollution in Poland" International Jour�nal of Environmental Research and Public Health 17(12): DOI: 10.3390/ijerph17124385.
- [6] I. R. Chowdhury, S. Chowdhury, M. A. J. Mazumder, and A. Al-Ahmed, (2022) “Removal of lead ions (Pb2+) from water and wastewater: a review on the low-cost ad�sorbents" Applied Water Science 12(8): DOI: 10.1007/s13201-022-01703-6.
- [7] E. Zhang, J. Wu, G. Wang, B. Zhang, and Y. Xie, (2016) “Efficient Fenton oxidation of Congo red dye by magnetic MgFe2O4 nanorods" Journal of Nanoscience and Nanotechnology 16(5): 4727–4732. DOI: 10.1166/jnn.2016.12627.
- [8] M. Tariq, M. Muhammad, J. Khan, A. Raziq, M. K. Uddin, A. Niaz, S. S. Ahmed, and A. Rahim, (2020) “Removal of Rhodamine B dye from aqueous solutions us�ing photo-Fenton processes and novel Ni-Cu@MWCNTs photocatalyst" Journal of Molecular Liquids 312: 113399. DOI: 10.1016/j.molliq.2020.113399.
- [9] E. T. Wahyuni, D. Siswanta, E. S. Kunarti, D. Supraba, and S. Budiraharjo, (2019) “Removal of Pb(II) ions in the aqueous solution by photo-Fenton method" Global Nest Journal 21(2): 180–186. DOI: 10.30955/gnj.002936.
- [10] S. S. Saric-Bosanac, A. K. Clark, V. Nguyen, A. Pan, F. Y. Chang, C. S. Li, and R. K. Sivamani, (2019) “Quantification of ultraviolet (UV) radiation in the shade and in direct sunlight" Dermatology Online Journal 25(7): 0–6. DOI: 10.5070/d3257044801.
- [11] M. Muruganandham, R. P. Suri, S. Jafari, M. Sillan�pää, G. J. Lee, J. J. Wu, and M. Swaminathan, (2014) “Recent developments in homogeneous advanced oxidation processes for water and wastewater treatment" Interna�tional Journal of Photoenergy 2014: DOI: 10.1155/2014/821674.
- [12] F. Z. Yehia, M. H. Helal, O. Ali, A. M. Elfadly, A. H. Mady, and A. A. Roshdy, (2013) “Catalytic degradation of phenol using different chelating agent at near neutral pH in modified-fenton process" Egyptian Journal of Chemistry 56(3): 199–212. DOI: 10.21608/ejchem.2013.1108.
- [13] H. Khorsandi, A. Mohammadi, F. Kariminejad, M. Haghighi, S. Karimzadeh, J. Khorsandi, and A. A. Aghapour, (2016) “Optimizing linear alkyl ben�zene sulfonate removal using Fenton oxidation process in Taguchi Method" Journal of Water Chemistry and Technology 38(5): 266–272. DOI: 10.3103/S1063455X16050040.
- [14] U. J. Ahile, R. A. Wuana, A. U. Itodo, R. Sha’Ato, and R. F. Dantas, (2020) “A review on the use of chelating agents as an alternative to promote photo-Fenton at neu�tral pH: Current trends, knowledge gap and future stud�ies" Science of the Total Environment 710: 134872. DOI: 10.1016/j.scitotenv.2019.134872.
- [15] Y. Pan, H. Guo, M. Zhou, Y. Zhang, Y. Tian, and W. Wang, (2019) “EDTA enhanced removal of sulfamet�hazine by pre-magnetized Fe0 without oxidant addition" Chemical Engineering Journal 372(February): 905–916. DOI: 10.1016/j.cej.2019.04.211.
- [16] N. Kishimoto, T. Kitamura, M. Kato, and H. Otsu, (2013) “Influence of Chelating Agents on Fenton-Type Re�action Using Ferrous Ion and Hypochlorous Acid" Jour�nal of Water and Environment Technology 11(1): 21–32. DOI: 10.2965/jwet.2013.21.
- [17] R. Harrison and D. Laxen. Lead pollution, causes and control. 28. 4. Champan and Hall, 1981, 313. DOI: 10.1016/0143-1471(82)90149-0.
- [18] X. Xue, K. Hanna, C. Despas, F. Wu, and N. Deng, (2009) “Effect of chelating agent on the oxidation rate of PCP in the magnetite/H2O2 system at neutral pH" Journal of Molecular Catalysis A: Chemical 311(1-2): 29–35. DOI: 10.1016/j.molcata.2009.06.016.
- [19] A. Naldoni, A. Schiboula, C. L. Bianchi, and D. H. Bremner, (2011) “Mineralisation of surfactants using ultrasound and the advanced fenton process" Water, Air, and Soil Pollution 215(1-4): 487–495. DOI: 10.1007/s11270-010-0493-y.
- [20] A. Verma, R. Kore, D. R. Corbin, and M. B. Shiflett, (2019) “Metal Recovery Using Oxalate Chemistry: A Technical Review" Industrial and Engineering Chem�istry Research 58(34): 15381–15393. DOI: 10.1021/acs.iecr.9b02598.
- [21] C. Kocks, J. Görtz, A. Holtz, M. Gausmann, and A. Jupke, (2020) “Electrochemical Crystallization Con�cept for Succinic Acid Reduces Waste Salt Production" Chemie-Ingenieur-Technik 92(3): 221–228. DOI: 10.1002/cite.201900088.
- [22] A. Jariyanorasade and S. Junyapoon, (2018) “Factors affecting the degradation of linear alkylbenzene sulfonate by TiO2 assisted photocatalysis and its kinetics" Envi�ronmentAsia 11(1): 45–60. DOI: 10.14456/ea.2018.4.
- [23] Y. H. Huang, Y. J. Huang, H. C. Tsai, and H. T. Chen, (2010) “Degradation of phenol using low concentration of ferric ions by the photo-Fenton process" Journal of the Taiwan Institute of Chemical Engineers 41: 699–704. DOI: 10.1016/j.jtice.2010.01.012.