Gaofeng Shi This email address is being protected from spambots. You need JavaScript enabled to view it.1,2, Guoying Wang1,2, Xuefu Chen1,2 and Chunlei Li1 1College of Petroleum and Chemical Engineering, Lanzhou University of Technology, Lanzhou 730050, P. R. China
2Analysis & Testing Center, Lanzhou University of Technology, Lanzhou 730050, P. R. China
Received:
March 11, 2012
Accepted:
September 12, 2012
Publication Date:
June 1, 2013
Download Citation:
||https://doi.org/10.6180/jase.2013.16.2.12
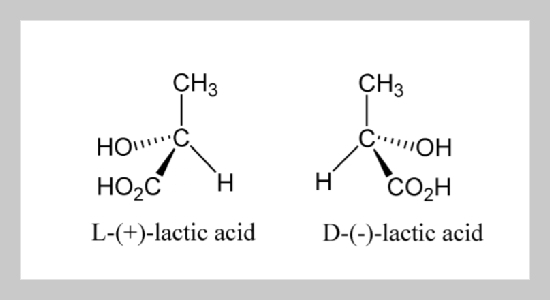
Optically pure L-(+)-lactic acid fermentation directly from leftover bits and pieces of potato starch (LBPPS) using an amylolytic pellet-form complex Rhizopus oryzae ASC081 was studied. The complex ASC081, which have the capability for the simultaneously saccharification and production of pure L-(+)-lactic acid, was mutagenized and screened from two parent Rhizopus oryzaes As1 and As2. In the ASC081 fermentation, the variation in temperature, inoculum size, and pH obviously affected the amylolytic capacity, the L-(+)-lactic acid production and the fungal biomass formation. The addition of phytic acid and bitter salt (an alternate name: magnesium sulfate) significantly stimulated the L-(+)-lactic acid fermentation process, and enabled complete bioconversion within 90 h. A condition, LBPPS concentration 140 g/L, pH 5.2, temperature 32 °C, inoculum size 15%, phytic acid addition 0.05 g/L and bitter salt addition 0.5 g/L, was favourable for the optically pure L-(+)-lactic acid production, resulting in the optically pure L-(+)-lactic acid yield of 0.12 g / 1.0 g LBPPS.ABSTRACT
Keywords:
Amylolytic, Rhizopus Oryze ASC081, Leftover Bits and Pieces of Potato Starch, Optically Pure L-Lactic Acid
REFERENCES