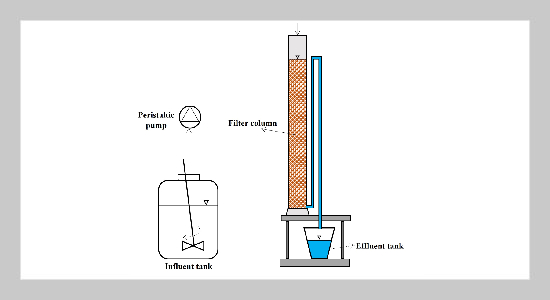
- [1] D. Cordell, J.-O. Drangert, and S. White, (2009) “The story of phosphorus: global food security and food for thought" Global environmental change 19(2): 292–305.
- [2] L. Ngatia, J. M. Grace III, D. Moriasi, and R. Taylor, (2019) “Nitrogen and phosphorus eutrophication in ma�rine ecosystems" Monitoring of marine pollution 1: 1–17.
- [3] N. Muisa, I. Nhapi, W. Ruziwa, and M. M. Manyuchi, (2020) “Utilization of alum sludge as adsorbent for phosphorus removal in municipal wastewater: A review" Jour�nal of Water Process Engineering 35: 1011.
- [4] G. Xu, T. Wang, Y. Wei, Y. Zhang, and J. Chen, (2022) “Fecal coliform distribution and health risk assessment in surface water in an urban-intensive catchment" Journal of Hydrology 604: 127204.
- [5] S. Liu, G. T. Daigger, B. Liu, W. Zhao, and J. Liu, (2020) “Enhanced performance of simultaneous carbon, nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor (AOA�SBR) system by alternating the cycle times" Bioresource technology 301: 122750.
- [6] C. Chaiwong, T. Koottatep, and C. Polprasert, (2021) “Effects of specific wavelengths of lights on performance of biofilm photobioreactor for treating septic tank effluent" Journal of Water Process Engineering 40: 101907.
- [7] P. Izadi, P. Izadi, and A. Eldyasti, (2020) “De�sign, operation and technology configurations for en�hanced biological phosphorus removal (EBPR) process: a review" Reviews in Environmental Science and Bio/Technology 19: 561–593.
- [8] C. Pratt, S. A. Parsons, A. Soares, and B. D. Martin, (2012) “Biologically and chemically mediated adsorption and precipitation of phosphorus from wastewater" Cur�rent opinion in Biotechnology 23(6): 890–896.
- [9] R. Bashar, K. Gungor, K. Karthikeyan, and P. Barak, (2018) “Cost effectiveness of phosphorus removal processes in municipal wastewater treatment" Chemosphere 197: 280–290.
- [10] C. C. Breda, M. B. Soares, R. F. R. Tavanti, D. G. Viana, O. da Silva Freddi, A. R. Piedade, D. Mahl, R. C. Traballi, and I. A. Guerrini, (2020) “Successive sewage sludge fertilization: Recycling for sustainable agriculture" Waste management 109: 38–50.
- [11] Q. Ping, X. Lu, Y. Li, and G. Mannina, (2020) “Effect of complexing agents on phosphorus release from chemical�enhanced phosphorus removal sludge during anaerobic fermentation" Bioresource technology 301: 122745.
- [12] S. Guida, G. Rubertelli, B. Jefferson, and A. Soares, (2021) “Demonstration of ion exchange technology for phosphorus removal and recovery from municipal wastew�ater" Chemical Engineering Journal 420: 129913.
- [13] G. Lee, S. Modarresi, and M. M. Benjamin, (2019) “Efficient phosphorus removal from MBR effluent with heated aluminum oxide particles (HAOPs)" Water re�search 159: 274–282.
- [14] R. Mohammadi, W. Tang, and M. Sillanpää, (2021) “A systematic review and statistical analysis of nutrient recovery from municipal wastewater by electrodialysis" Desalination 498: 114626.
- [15] J. T. Bunce, E. Ndam, I. D. Ofiteru, A. Moore, and D. W. Graham, (2018) “A review of phosphorus removal technologies and their applicability to small-scale domestic wastewater treatment systems" Frontiers in Environ�mental Science 6: 8.
- [16] T. Prot, V. Nguyen, P. Wilfert, A. Dugulan, K. Goubitz, D. De Ridder, L. Korving, P. Rem, A. Bouderbala, G. J. Witkamp, et al., (2019) “Magnetic separation and characterization of vivianite from digested sewage sludge" Separation and Purification Technology 224: 564–579.
- [17] W. Huang, Y. Zhang, and D. Li, (2017) “Adsorptive removal of phosphate from water using mesoporous mate�rials: A review" Journal of Environmental Manage�ment 193: 470–482.
- [18] J. Nie, Q. Wang, S. Gao, C. S. Poon, Y. Zhou, and J.-s. Li, (2021) “Novel recycling of incinerated sewage sludge ash (ISSA) and waste bentonite as ceramsite for Pb-containing wastewater treatment: Performance and mechanism" Journal of Environmental Management 288: 112382.
- [19] A. Gopinath, G. Divyapriya, V. Srivastava, A. Laiju, P. Nidheesh, and M. S. Kumar, (2021) “Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment" Environmental Research 194: 110656.
- [20] Y. Cai, C. Li, D. Wu, W. Wang, F. Tan, X. Wang, P. K. Wong, and X. Qiao, (2017) “Highly active MgO nanoparticles for simultaneous bacterial inactivation and heavy metal removal from aqueous solution" Chemical Engineering Journal 312: 158–166.
- [21] P. K. Stoimenov, R. L. Klinger, G. L. Marchin, and K. J. Klabunde, (2002) “Metal oxide nanoparticles as bactericidal agents" Langmuir 18(17): 6679–6686.
- [22] A. A. Aryee, F. M. Mpatani, R. Han, X. Shi, and L. Qu, (2021) “A review on adsorbents for the remediation of wastewater: Antibacterial and adsorption study" Jour�nal of Environmental Chemical Engineering 9(6): 106907.
- [23] M. Kumari and S. Gupta, (2022) “Occurrence and ex�posure to trihalomethanes in drinking water: a systematic review and meta-analysis" Exposure and Health 14(4): 915–939.
- [24] J. Qin, Z. Zhu, Z. Chen, X. Wang, Y. Zhang, and H. Chen, (2023) “Synthesis, characterization and applica�tion of dewatered municipal sludge-based creamsite and its phosphorus adsorption characteristics" Journal of Cleaner Production 391: 136216.
- [25] C. Wan, S. Ding, C. Zhang, X. Tan, W. Zou, X. Liu, and X. Yang, (2017) “Simultaneous recovery of nitrogen and phosphorus from sludge fermentation liquid by zeolite adsorption: Mechanism and application" Separation and Purification Technology 180: 1–12.
- [26] E. P. Kuncoro, T. Soedarti, T. W. C. Putranto, H. Darmokoesoemo, N. R. Abadi, and H. S. Kusuma, (2018) “Characterization of a mixture of algae waste�bentonite used as adsorbent for the removal of Pb2+ from aqueous solution" Data in brief 16: 908–913.
- [27] E. P. Kuncoro, D. R. M. Isnadina, H. Darmokoe�soemo, F. Dzembarahmatiny, and H. S. Kusuma, (2018) “Characterization and isotherm data for adsorption of Cd2+ from aqueous solution by adsorbent from mixture of bagasse-bentonite" Data in brief 16: 354–360.
- [28] Y. A. Neolaka, A. A. Riwu, U. O. Aigbe, K. E. Ukhure�bor, R. B. Onyancha, H. Darmokoesoemo, and H. S. Kusuma, (2022) “Potential of activated carbon from vari�ous sources as a low-cost adsorbent to remove heavy metals and synthetic dyes" Results in Chemistry: 100711.
- [29] X. Liu, S. Yang, S. Liu, and Y. Yang, (2021) “Perfor�mance and mechanism of phosphorus removal by slag ceramsite filler" Process Safety and Environmental Protection 148: 858–866.
- [30] S. Kang, J.-H. Choi, J.-G. Park, and K. Baek, (2019) “Pellet adsorbent derived from molasses and dewatered alum sludge for arsenic removal" Journal of CO2 Uti�lization 33: 31–36.
- [31] C. Liang, S. Lin, and G. Liu, (2021) “Study on the prepa�ration of biochar ceramsite based on sewage sludge and the characterization of its properties" Applied Sciences 11(12): 5522.
- [32] Q. Shao, Y. Zhang, Z. Liu, L. Long, Z. Liu, Y. Chen, X.-M. Hu, M. Lu, and L.-Z. Huang, (2022) “Phospho�rus and nitrogen recovery from wastewater by ceramsite: Adsorption mechanism, plant cultivation and sustainabil�ity analysis" Science of the Total Environment 805: 150288.
- [33] A. W. W. A. (APHA) and W. E. F. (WEF). Stan�dard Methods for Examination of Water and Wastewater. United Book Press Inc, 2012.
- [34] Y. A. Neolaka, Y. Lawa, J. N. Naat, A. A. Riwu, M. Iqbal, H. Darmokoesoemo, and H. S. Kusuma, (2020) “The adsorption of Cr (VI) from water samples using graphene oxide-magnetic (GO-Fe3O4) synthesized from natural cellulose-based graphite (kusambi wood or Schle�ichera oleosa): Study of kinetics, isotherms and thermody�namics" Journal of Materials Research and Technol�ogy 9(3): 6544–6556.
- [35] S. Dai, Q. Wen, F. Huang, Y. Bao, X. Xi, Z. Liao, J. Shi, C. Ou, and J. Qin, (2022) “Preparation and applica�tion of MgO-loaded tobermorite to simultaneously remove nitrogen and phosphorus from wastewater" Chemical Engineering Journal 446: 136809.
- [36] X. Liu, Y. Wang, R. L. Smith, J. Fu, and X. Qi, (2021) “High-capacity structured MgO-Co adsorbent for removal of phosphorus from aqueous solutions" Chemical Engi�neering Journal 426: 131381.
- [37] Y. Fang, A. Ali, Y. Gao, P. Zhao, R. Li, X. Li, J. Liu, Y. Luo, Y. Peng, H. Wang, et al., (2022) “Preparation and characterization of MgO hybrid biochar and its mech�anism for high efficient recovery of phosphorus from aque�ous media" Biochar 4(1): 40.
- [38] F. Xie, F. Wu, G. Liu, Y. Mu, C. Feng, H. Wang, and J. P. Giesy, (2014) “Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite" Environmental Science & Technology 48(1): 582–590.
- [39] C. Barca, D. Meyer, M. Liira, P. Drissen, Y. Comeau, Y. Andrès, and F. Chazarenc, (2014) “Steel slag fil�ters to upgrade phosphorus removal in small wastewater treatment plants: removal mechanisms and performance" Ecological engineering 68: 214–222.
- [40] I. Blanco, P. Molle, L. E. S. de Miera, and G. Ansola, (2016) “Basic oxygen furnace steel slag aggregates for phosphorus treatment. Evaluation of its potential use as a substrate in constructed wetlands" Water research 89: 355–365.
- [41] T. Park, V. Ampunan, S. Maeng, and E. Chung, (2017) “Application of steel slag coated with sodium hydroxide to enhance precipitation-coagulation for phosphorus re�moval" Chemosphere 167: 91–97.
- [42] J. Yu, W. Liang, L. Wang, F. Li, Y. Zou, and H. Wang, (2015) “Phosphate removal from domestic wastewater us�ing thermally modified steel slag" Journal of Environ�mental Sciences 31: 81–88.
- [43] F. C. P. Masim, C.-H. Tsai, Y.-F. Lin, M.-L. Fu, M. Liu, F. Kang, and Y.-F. Wang, (2019) “Synergistic effect of PANI–ZrO2 composite as antibacterial, anti-corrosion, and phosphate adsorbent material: synthesis, characteri�zation and applications" Environmental technology 40(2): 226–238.
- [44] L.-S. Lin, C.-G. Niu, N. Tang, C. Liang, X.-X. Lv, H. Guo, L. Zhang, Y.-Y. Yang, and H.-Y. Liu, (2020) “Lanthanum hydroxides modified poly (epichlorohydrin)- ethylenediamine composites for highly efficient phosphate removal and bacteria disinfection" Colloids and Sur�faces A: Physicochemical and Engineering Aspects 588: 124344.
- [45] C. T. Vu and T. Wu, (2020) “Magnetic porous NiLa�Layered double oxides (LDOs) with improved phosphate adsorption and antibacterial activity for treatment of sec�ondary effluent" Water Research 175: 115679.
- [46] H. Wang, J. Xu, Y. Liu, and L. Sheng, (2021) “Prepara�tion of ceramsite from municipal sludge and its application in water treatment: A review" Journal of environmen�tal management 287: 112374.
- [47] U. Baig, M. Faizan, and M. Sajid, (2021) “Effective removal of hazardous pollutants from water and deactiva�tion of water-borne pathogens using multifunctional syn�thetic adsorbent materials: a review" Journal of Cleaner Production 302: 126735.
- [48] B. Michen, J. Fritsch, C. Aneziris, and T. Graule, (2013) “Improved virus removal in ceramic depth filters modified with MgO" Environmental science & technology 47(3): 1526–1533.
- [49] M. bibinitperiod Eddy, M. Abu-Orf, G. Bowden, F. L. Burton, W. Pfrang, H. D. Stensel, G. Tchobanoglous, R. Tsuchihashi, and A. (Firm). Wastewater engineering: treatment and resource recovery. McGraw Hill Educa�tion, 2014.
- [50] J. B. Quispe, L. Campos, O. Mašek, and A. Bogush, (2022) “Use of biochar-based column filtration systems for greywater treatment: A systematic literature review" Journal of Water Process Engineering 48: 102908.
- [51] S. Kataki, H. West, M. Clarke, and D. C. Baruah, (2016) “Phosphorus recovery as struvite from farm, mu�nicipal and industrial waste: Feedstock suitability, meth�ods and pre-treatments" Waste Management 49: 437–454.
- [52] J.-Y. Lin, D. Li, M. Kim, I. Lee, H. Kim, and C.-P. Huang, (2021) “Process optimization for the synthesis of ceramsites in terms of mechanical strength and phosphate adsorption capacity" Chemosphere 278: 130239.
- [53] M. Shirazinezhad, M. Faghihinezhad, M. Baghdadi, and M. Ghanbari, (2021) “Phosphate removal from mu�nicipal effluent by a porous MgO-expanded graphite com�posite as a novel adsorbent: Evaluation of seawater as a natural source of magnesium ions" Journal of Water Process Engineering 43: 102232.
- [54] G. Selvaraju and N. K. A. Bakar, (2017) “Production of a new industrially viable green-activated carbon from Artocarpus integer fruit processing waste and evaluation of its chemical, morphological and adsorption properties" Journal of cleaner production 141: 989–999.
- [55] S. Pap, C. Kirk, B. Bremner, M. T. Sekulic, L. Shearer, S. W. Gibb, and M. A. Taggart, (2020) “Low-cost chitosan-calcite adsorbent development for potential phos�phate removal and recovery from wastewater effluent" Water research 173: 115573.
- [56] A. S. Mahmoud, M. K. Mostafa, and M. Nasr, (2019) “Regression model, artificial intelligence, and cost es�timation for phosphate adsorption using encapsulated nanoscale zero-valent iron" Separation Science and Technology 54(1): 13–26.
- [57] R. Li, J. J. Wang, B. Zhou, M. K. Awasthi, A. Ali, Z. Zhang, A. H. Lahori, and A. Mahar, (2016) “Recovery of phosphate from aqueous solution by magnesium oxide decorated magnetic biochar and its potential as phosphate�based fertilizer substitute" Bioresource technology 215: 209–214.
- [58] E. P. Kuncoro, D. R. M. Isnadina, H. Darmokoesoemo, O. R. Fauziah, and H. S. Kusuma, (2018) “Charac�terization, kinetic, and isotherm data for adsorption of Pb2+ from aqueous solution by adsorbent from mixture of bagasse-bentonite" Data in brief 16: 622–629.
- [59] L. L. Cabral, I. C. Pereira, F. Perretto, A. Nagalli, R. C. P. Rizzo-Domingues, F. H. Passig, and K. Q. de Carvalho, (2021) “Adsorption and desorption of phos�phate onto chemically and thermochemically pre-activated red ceramic waste: Characteristics, batch studies, and mechanisms" Journal of Environmental Chemical Engineering 9(6): 106695.