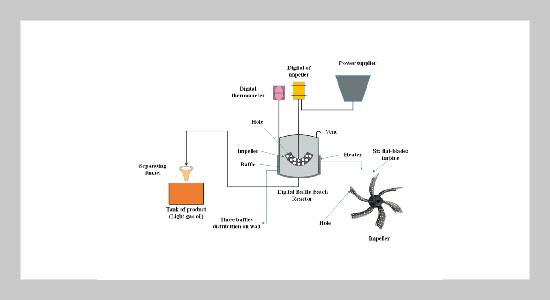
- [1] L. A. Khamees, A. A. A. A. Alrazzaq, and J. I. Humadi, (2022) “Different methods for determination of shale volume for Yamama formation in an oil field in southern Iraq" Materials Today: Proceedings 57: 586–594. DOI: https://doi.org/10.1016/j.matpr.2022.01.455.
- [2] V. Vivoda, (2009) “Diversification of oil import sources and energy security: A key strategy or an elusive objective?" Energy Policy 37(11): 4615–4623. DOI: https://doi.org/10.1016/j.enpol.2009.06.007.
- [3] A. A. Umar, I. B. M. Saaid, A. A. Sulaimon, and R. B. M. Pilus, (2018) “A review of petroleum emulsions and recent progress on water-in-crude oil emulsions stabilized by natural surfactants and solids" Journal of Petroleum Science and Engineering 165: 673–690. DOI: https://doi.org/10.1016/j.petrol.2018.03.014.
- [4] J. I. Humadi, S. A. Gheni, S. M. Ahmed, G. H. Abdullah, A. N. Phan, and A. P. Harvey, (2021) “Fast, non-extractive, and ultradeep desulfurization of diesel in an oscillatory baffled reactor" Process Safety and Environmental Protection 152: 178–187. DOI: https://doi.org/10.1016/j.psep.2021.05.028.
- [5] S. Subhan, A. Ur Rahman, M. Yaseen, H. Ur Rashid, M. Ishaq, M. Sahibzada, and Z. Tong, (2019) “Ultrafast and highly efficient catalytic oxidative desulfurization of dibenzothiophene at ambient temperature over low Mn loaded Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using NaClO as oxidant" Fuel 237: 793–805. DOI: https://doi.org/10.1016/j.fuel.2018.10.067.
- [6] A. A. Aabid, S. A. Jafar, M. Obaid Ahmed, and J. I. Humadi, (2021) “WITHDRAWN: Inhibitory effect of ceftriaxone sodium on corrosion of copper in 1M nitric acid" Materials Today: Proceedings: DOI: https://doi.org/10.1016/j.matpr.2021.05.345.
- [7] S. A. Jafar, A. A. Aabid, and J. I. Humadi, (2022) “Corrosion behavior of carbon steel in 1 M, 2 M, and 3 M HCl solutions" Materials Today: Proceedings 57: 412–417. DOI: https://doi.org/10.1016/j.matpr.2021.12.295.
- [8] S. A. Jafar, A. A. Aabid, G. H. Abdul Razzaq, and J. I. Humadi, (2022) “Sodium nitrate as a corrosion inhibitor of carbon steel in various concentrations of hydrochloric acid solution" Journal of New Materials for Electrochemical Systems 25(1): 32–37. DOI: 10.14447/JNMES.V25I1.A05.
- [9] G. Khare, (2001) “Desulfurization process and novel bimetallic sorbent systems for same" Patent:
- [10] A. Seeberger and A. Jess, (2010) “Desulfurization of diesel oil by selective oxidation and extraction of sulfur compounds by ionic liquids—a contribution to a competitive process design" Green Chemistry 12(4): 602–60. DOI: 10.1039/b918724c.
- [11] Y. Muhammad, A. Shoukat, A. U. Rahman, H. U. Rashid, and W. Ahmad, (2018) “Oxidative desulfurization of dibenzothiophene over Fe promoted Co–Mo/Al2O3 and Ni–Mo/Al2O3 catalysts using hydrogen peroxide and formic acid as oxidants" Chinese Journal of Chemical Engineering 26(3): 593–600. DOI: 10.1016/j.cjche.2017.05.015.
- [12] J. Cecilia, A. Infantes-Molina, E. Rodríguez-Castellón, and A. Jiménez-López, (2009) “Dibenzothiophene hydrodesulfurization over cobalt phosphide catalysts prepared through a new synthetic approach: Effect of the support" Applied Catalysis B: Environmental 92(1-2): 100–113. DOI: 10.1016/j.apcatb.2009.07.017.
- [13] Y. Muhammad and C. Li, (2011) “Dibenzothiophene hydrodesulfurization using in situ generated hydrogen over Pd promoted alumina-based catalysts" Fuel Processing Technology 92(3): 624–630. DOI: 10.1016/j.fuproc.2010.11.021.
- [14] S. A. Jafar, A. T. Nawaf, and J. I. Humadi. “Improving the extraction of sulfur-containing compounds from fuel using surfactant material in a digital baffle reactor”. In: 42. Cited by: 8. 2021, 1777–1783. DOI: 10.1016/j.matpr.2020.11.821.
- [15] W. Wan Abu Bakar, A. Rusmidah, A. Abdul Aziz, and W. Wan Mokhtar. “The role of molybdenum oxide based catalysts on oxidative desulfurization of diesel fuel,” in: Modern Chem. Appl. 2015.
- [16] J. I. Humadi, Y. S. Issa, D. Y. Aqar, M. A. Ahmed, H. H. A. Alak, and I. M. Mujtaba, (2023) International Journal of Chemical Reactor Engineering 21(6): 727–741. DOI: doi:10.1515/ijcre-2022-0046.
- [17] M. I. Fathi, J. I. Humadi, Q. A. Mahmood, A. T. Nawaf, and R. S. Ayoub. “Improvement of Design Synthetic Nano-Catalysts for Performance Enhancement of Oxidative Desulfurization Using Batch Reactor”. In: 2660. Cited by: 3; All Open Access, Bronze Open Access. 2022. DOI: 10.1063/5.0109089.
- [18] S.-W. Li, R.-M. Gao, R.-L. Zhang, and J.-s. Zhao, (2016) “Template method for a hybrid catalyst material POM@MOF-199 anchored on MCM-41: Highly oxidative desulfurization of DBT under molecular oxygen" Fuel 184: 18–27. DOI: https://doi.org/10.1016/j.fuel.2016.06.132.
- [19] G. G. Zeelani, A. Ashrafi, A. Dhakad, G. Gupta, and S. L. Pal, (2016) “Catalytic oxidative desulfurization of liquid fuels: a review" International Research Journal of Engineering and Technology 3: 331–336.
- [20] G. S. Ahmed, J. I. Humadi, and A. A. Aabid, (2021) “Mathematical model, simulation and scale up of batch reactor used in oxidative desulfurization of kerosene" Iraqi Journal of Chemical and Petroleum Engineering 22(3): 11–17.
- [21] J. I. Humadi, S. A. Gheni, S. M. R. Ahmed, and A. Harvey, (2022) “Dimensionless evaluation and kinetics of rapid and ultradeep desulfurization of diesel fuel in an oscillatory baffled reactor" RSC Advances 12(23): 14385–14396. DOI: 10.1039/d2ra01663j.
- [22] R. Ghubayra, C. Nuttall, S. Hodgkiss, M. Craven, E. F. Kozhevnikova, and I. V. Kozhevnikov, (2019) “Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids" Applied Catalysis B: Environmental 253: 309–316. DOI: 10.1016/j.apcatb.2019.04.063.
- [23] A. T. Jarullah, M. A. Ahmed, B. A. Al-Tabbakh, and I. M. Mujtaba, (2023) “Design of a new synthetic nanocatalyst resulting high fuel quality based on multiple supports: Experimental investigation and modeling" Chemical Product and Process Modeling 18(2): 265–293. DOI: 10.1515/cppm-2021-0073.
- [24] A. T. Jarullah, B. A. Al-Tabbakh, M. A. Ahmed, S. m. A. Hameed, and I. M. Mujtaba, (2022) “Design of Novel Synthetic Iron Oxide Nano-Catalyst over Homemade Nano-Alumina for an Environmentally Friendly Fuel: Experiments and Modelling" Petroleum and Coal 64(4): 917–937.
- [25] R. Hemrajani and G. Tatterson. Mechanically Stirred Vessels. Handbook of Industrial Mixing: Science and Practice (Hoboken: John Wiley & Sons Inc.) 2003, 345–390.
- [26] J. I. Humadi, G. H. A. Razzaq, L. A. Khamees, M. A. Ahmed, and L. I. Saeed, (2023) “Improved Kerosene Quality with the Use of a Gamma Alumina Nanoparticles Supported Zinc Oxide Catalyst in a Digital Batch Baffled Reactor: Experiments and Process Modelling" Korean Chemical Engineering Research 61(2): 226–233.
- [27] J.-P. Torré, D. F. Fletcher, T. Lasuye, and C. Xuereb, (2007) “An experimental and computational study of the vortex shape in a partially baffled agitated vessel" Chemical Engineering Science 62(7): 1915–1926. DOI: 10.1016/j.ces.2006.12.020.
- [28] J. I. Humadi, A. T. Nawaf, A. T. Jarullah, M. A. Ahmed, S. A. Hameed, and I. M. Mujtaba, (2023) “Design of new nano-catalysts and digital basket reactor for oxidative desulfurization of fuel: Experiments and modelling" Chemical Engineering Research and Design 190: 634–650. DOI: 10.1016/j.cherd.2022.12.043.
- [29] Y. Kamla, M. Bouzit, H. Ameur, M. I. Arab, and A. Hadjeb, (2017) “Effect of the Inclination of Baffles on the Power Consumption and Fluid Flows in a Vessel Stirred by a Rushton Turbine" Chinese Journal of Mechanical Engineering (English Edition) 30(4): 1008–1016. DOI: 10.1007/s10033-017-0158-5.
- [30] A. Mohammad-Khah and R. Ansari, (2009) “Activated charcoal: Preparation, characterization and applications: A review article" International Journal of ChemTech Research 1(4): 859–864.
- [31] G. Yu, S. Lu, H. Chen, and Z. Zhu, (2005) “Oxidative desulfurization of diesel fuels with hydrogen peroxide in the presence of activated carbon and formic acid" Energy and Fuels 19(2): 447–452. DOI: 10.1021/ef049760b.
- [32] J. I. Humadi, A. T. Nawaf, L. A. Khamees, Y. A. AbdAlhussain, H. F. Muhsin, M. A. Ahmed, and M. M. Ahmed, (2022) “Development of new effective activated carbon supported alkaline adsorbent used for removal phenolic compounds from refinery waste water in a fixed bed adsorption column": DOI: 10.21203/rs.3.rs-2210259/v1.
- [33] K.-G. Haw, W. A. W. A. Bakar, R. Ali, J.-F. Chong, and A. A. A. Kadir, (2010) “Catalytic oxidative desulfurization of diesel utilizing hydrogen peroxide and functionalized-activated carbon in a biphasic dieselacetonitrile system" Fuel Processing Technology 91(9): 1105–1112. DOI: 10.1016/j.fuproc.2010.03.021.
- [34] W. N. W. Abdullah, W. A. W. A. Bakar, N. H. Abdullah, W. N. A. W. Mokhtar, S. J. M. Rosid, and N. M. Shukri. “Effect of activated carbon supported Ce/Fe based catalyst for catalytic oxidative desulfurization of Malaysian diesel fuel”. In: 2068. Cited by: 3. 2019. DOI: 10.1063/1.5089374.
- [35] A. R. Yacob, N. M. Mustapha, M. K. A. A. Mustajab, and H. M. Al Swaidan. “Physical activation of Saudi Arabia date palm tree’s foliar, frond and thorn”. In: Cited by: 1. 2010, 511–517. DOI: 10.1115/1.859544.paper80.
- [36] D. Kalderis, S. Bethanis, P. Paraskeva, and E. Diamadopoulos, (2008) “Production of activated carbon from bagasse and rice husk by a single-stage chemical activation method at low retention times" Bioresource Technology 99(15): 6809–6816. DOI: 10.1016/j.biortech.2008.01.041.
- [37] J. Hayashi, T. Horikawa, I. Takeda, K. Muroyama, and F. Nasir Ani, (2002) “Preparing activated carbon from various nutshells by chemical activation with K2CO3" Carbon 40(13): 2381–2386. DOI: 10.1016/S0008-6223(02)00118-5.
- [38] T. Watari, H. Tsubira, T. Torikai, M. Yada, and S. Furuta, (2003) “Preparation of porous carbon/silica composites from rice husk powder" Journal of Ceramic Processing Research 4(4): 177–180.
- [39] K. I. Hamad, J. I. Humadi, Y. S. Issa, S. A. Gheni, M. A. Ahmed, and A. A. Hassan, (2022) “Enhancement of activity and lifetime of nano-iron oxide catalyst for environmentally friendly catalytic phenol oxidation process" Cleaner Engineering and Technology 11: DOI: 10.1016/j.clet.2022.100570.
- [40] W. Li, K. Yang, J. Peng, L. Zhang, S. Guo, and H. Xia, (2008) “Effects of carbonization temperatures on characteristics of porosity in coconut shell chars and activated carbons derived from carbonized coconut shell chars" Industrial Crops and Products 28(2): 190–198. DOI: 10.1016/j.indcrop.2008.02.012.
- [41] A. IkhtiarBakti and P. L. Gareso, (2018) “Characterization of active carbon prepared from coconuts shells using FTIR, XRD and SEM techniques" J. Ilm. Pendidik. Fis. Al-Biruni 7: 33–39.
- [42] C. Saka, (2012) “BET, TG-DTG, FT-IR, SEM, iodine number analysis and preparation of activated carbon from acorn shell by chemical activation with ZnCl2" Journal of Analytical and Applied Pyrolysis 95: 21–24. DOI: 10.1016/j.jaap.2011.12.020.
- [43] A. A. Aabid, J. I. Humadi, G. S. Ahmed, A. T. Jarullah, M. A. Ahmed, and W. S. Abdullah, (2023) “Enhancement of Desulfurization Process for Light Gas Oil Using New Zinc Oxide Loaded Over Alumina Nanocatalyst" Applied Science and Engineering Progress 16(3): DOI: 10.14416/j.asep.2023.02.007.
- [44] R. J. Algawi, J. I. Humadi, and L. A. Khamees, (2023) “Experimental study of the impact of metal (iron, copper and aluminum) surface and light exposure on gum formation in Iraqi automotive gasoline" Petroleum Science and Technology: 1–12.
- [45] G. H. A. Razzaq, K. I. Hamad, and J. I. Humadi. “Silver Nanoparticles for Ultrasonic Assisted Synthesis of Oxidant Agents in Micro-Reactor: Kinetic Analysis and Process Intensification”. In: Materials Science Forum. 1083. Trans Tech Publ. 2023, 23–32.
- [46] N. Ghorbani and G. Moradi, (2019) “Oxidative desulfurization of model and real oil samples using Mo supported on hierarchical alumina–silica: Process optimization by Box–Behnken experimental design" Chinese Journal of Chemical Engineering 27(11): 2759–2770. DOI: 10.1016/j.cjche.2019.01.037.
- [47] N. K. Ibrahim and S. K. Aljanabi, (2015) “Desulfurization and kinetic study of diesel fuel by batch adsorption on activated carbon" Engineering and Technology Journal 33(8): 16.
- [48] J. I. Humadi, S. A. Jafar, N. S. Ali, M. A. Ahmed, M. J. Mzeed, R. J. Al-Salhi, N. M. C. Saady, H. S. Majdi, S. Zendehboudi, and T. M. Albayati, (2023) “Recovery of fuel from real waste oily sludge via a new eco-friendly surfactant material used in a digital baffle batch extraction unit" Scientific Reports 13(1): DOI: 10.1038/s41598-023-37188-9.
- [49] A. T. Nawaf, J. I. Humadi, A. T. Jarullah, M. A. Ahmed, S. A. Hameed, and I. M. Mujtaba, (2023) “Design of Nano-Catalyst for Removal of Phenolic Compounds from Wastewater by Oxidation Using Modified Digital Basket Baffle Batch Reactor: Experiments and Modeling" Processes 11(7): DOI: 10.3390/pr11071990.
- [50] R. Tetrisyanda, A. Wiguno, R. R. Ginting, M. C. Dzikrillah, and G. Wibawa, (2018) “Residue oil desulfurization using oxidation and extraction method" Indonesian Journal of Chemistry 18(2): 242–249. DOI: 10.22146/ijc.26722.