- [1] S. Zhao, B. Wang, Z. Zhang, X. Zhang, S. He, and H. Yu, (2022) “First-principles computational insights into lithium battery cathode materials" Electrochemical Energy Reviews: 1–31. DOI: 10.1007/s41918-021-00115-5.
- [2] M. S. Houache, C.-H. Yim, Z. Karkar, and Y. AbuLebdeh, (2022) “On the Current and Future Outlook of Battery Chemistries for Electric Vehicles—Mini Review" Batteries 8(7): 70. DOI: 10.3390/batteries8070070.
- [3] M. Xiao, L. Wang, F. Ji, and F. Shi, (2016) “Converting chemical energy to electricity through a three-jaw mini-generator driven by the decomposition of hydrogen peroxide" ACS Applied Materials & Interfaces 8(18): 11403–11411. DOI: 10.1021/acsami.6b00550.
- [4] Z. Ahsan, B. Ding, Z. Cai, C. Wen, W. Yang, Y. Ma, S. Zhang, G. Song, and M. S. Javed, (2021) “Recent progress in capacity enhancement of LiFePO4 cathode for Li-ion batteries" Journal of Electrochemical Energy Conversion and Storage 18(1): 010801. DOI: 10.1115/1.4047222.
- [5] L.-X. Yuan, Z.-H. Wang, W.-X. Zhang, X.-L. Hu, J.-T. Chen, Y.-H. Huang, and J. B. Goodenough, (2011) “Development and challenges of LiFePO4 cathode material for lithium-ion batteries" Energy & Environmental Science 4(2): 269–284. DOI: 10.1039/C0EE00029A.
- [6] G. Xu, K. Zhong, J.-M. Zhang, and Z. Huang, (2015) “First-principles study of structural, electronic and Li-ion diffusion properties of N-doped LiFePO4 (010) surface" Solid State Ionics 281: 1–5. DOI: 10.1016/j.ssi.2015.08.013.
- [7] T. Bashir, S. A. Ismail, Y. Song, R. M. Irfan, S. Yang, S. Zhou, J. Zhao, and L. Gao, (2021) “A review of the energy storage aspects of chemical elements for lithiumion based batteries" Energy Mater 1: 100019. DOI: 10.20517/energymater.2021.20.
- [8] H. Djodi, E. Kartini, et al. “The improving conductivity of LiFePO4 by optimizing the calendaring process”. In: IOP Conference Series: Materials Science and Engineering. 432. 1. IOP Publishing. 2018, 012059. DOI: 10.1088/1757-899X/432/1/012059.
- [9] S. Sarkar and S. Mitra, (2014) “Carbon coated submicron sized-LiFePO4: improved high rate performance lithium battery cathode" Energy Procedia 54: 718–724. DOI: 10.1016/j.egypro.2014.07.312.
- [10] H. Raj and A. Sil, (2018) “Effect of carbon coating on electrochemical performance of LiFePO4 cathode material for Li-ion battery" Ionics 24: 2543–2553. DOI: 10.1007/s11581-017-2423-0.
- [11] Y. Gao, K. Xiong, H. Zhang, and B. Zhu, (2021) “Effect of Ru doping on the properties of LiFePO4/C cathode materials for lithium-ion batteries" ACS omega 6(22): 14122–14129. DOI: 10.1021/acsomega.1c00595.
- [12] S.-X. Zhao, H. Ding, Y.-C. Wang, B.-H. Li, and C.-W. Nan, (2013) “Improving rate performance of LiFePO4 cathode materials by hybrid coating of nano-Li3PO4 and carbon" Journal of Alloys and Compounds 566: 206–211. DOI: 10.1016/j.jallcom.2013.03.041.
- [13] A. B. Yaroslavtsev and I. A. Stenina, (2020) “Carbon coating of electrode materials for lithium-ion batteries" Surface Innovations 9(2–3): 92–110. DOI: 10.1680/jsuin.20.00044.
- [14] Y. Lu, Q. Zhang, and J. Chen, (2019) “Recent progress on lithium-ion batteries with high electrochemical performance" Science China Chemistry 62: 533–548. DOI: 10.1007/s11426-018-9410-0.
- [15] X. Yan, L. Xin, H. Wang, C. Cao, and S. Sun, (2019) “Synergetic effect of Na-doping and carbon coating on the electrochemical performances of Li3- xNa xV2 (PO4)3/C as cathode for lithium-ion batteries" RSC advances 9(15): 8222–8229. DOI: 10.1039/C8RA10646K.
- [16] R. C. K. Reddy, X. Lin, A. Zeb, and C.-Y. Su, (2022) “Metal–organic frameworks and their derivatives as cathodes for lithium-ion battery applications: a review" Electrochemical Energy Reviews: 1–36. DOI: 10.1007/s41918-021-00101-x.
- [17] J. Conder, C. Vaulot, C. Marino, C. Villevieille, and C. M. Ghimbeu, (2019) “Chitin and chitosan—Structurally related precursors of dissimilar hard carbons for Na-ion battery" ACS Applied Energy Materials 2(7): 4841–4852. DOI: 10.1021/acsaem.9b00545.
- [18] R. Vinodh, Y. Sasikumar, H.-J. Kim, R. Atchudan, and M. Yi, (2021) “Chitin and chitosan based biopolymer derived electrode materials for supercapacitor applications: A critical review" Journal of Industrial and Engineering Chemistry 104: 155–171. DOI: 10.1016/j.jiec.2021.08.019.
- [19] X. Yang, D. Liu, X. Xu, X. He, and J. Xie, (2013) “Mechanism and kinetic studies on the synthesis of LiFePO4 via solid-state reactions" CrystEngComm 15(48): 10648–10656. DOI: 10.1039/C3CE41063C.
- [20] F. Yu, L. Zhang, L. Lai, M. Zhu, Y. Guo, L. Xia, P. Qi, G. Wang, and B. Dai, (2015) “High electrochemical performance of LiFePO4 cathode material via in-situ microwave exfoliated graphene oxide" Electrochimica Acta 151: 240–248. DOI: 10.1016/j.electacta.2014.11.014.
- [21] D. Jiang, X. Zhang, T. Zhao, B. Liu, R. Yang, H. Zhang, T. Fan, and F. Wang, (2020) “An improved synthesis of iron phosphate as a precursor to synthesize lithium iron phosphate" Bulletin of Materials Science 43(1): 50. DOI: 10.1007/s12034-019-1994-y.
- [22] J. Ying, M. Lei, C. Jiang, C. Wan, X. He, J. Li, L. Wang, and J. Ren, (2006) “Preparation and characterization of high-density spherical Li0. 97Cr0. 01FePO4/C cathode material for lithium ion batteries" Journal of Power Sources 158(1): 543–549. DOI: 10.1016/j.jpowsour. 2005.08.045.
- [23] S. E. bibinitperiod Services. Resistivity of Semiconductors by Four Probe Method at Different Temperatures. https://www.iiserkol.ac.in/~ph324/ExptManuals/ResistivityFourProbe.pdf.
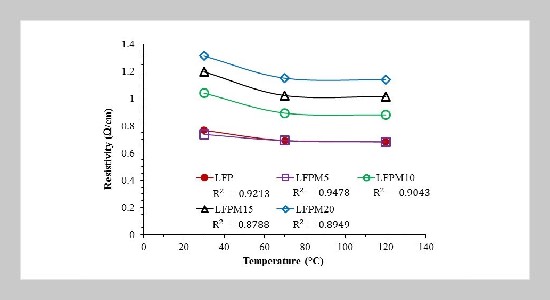
- [24] M. Alipour, C. Ziebert, F. V. Conte, and R. Kizilel, (2020) “A review on temperature-dependent electrochemical properties, aging, and performance of lithium-ion cells" Batteries 6(3): 35. DOI: 10.3390/batteries6030035.
- [25] M. F. I. M. Razi, Z. H. C. Daud, Z. Asus, I. I. Mazali, M. I. Ardani, and M. K. A. Hamid, (2021) “A review of internal resistance and temperature relationship, state of health and thermal runaway for lithium-ion battery beyond normal operating condition" Journal of Advanced Research in Fluid Mechanics and Thermal Sciences 88(2): 123–132. DOI: 10.37934/arfmts.88.2.123132.
- [26] H. Raj and A. Sil, (2019) “PEDOT: PSS coating on pristine and carbon coated LiFePO4 by one-step process: the study of electrochemical performance" Journal of Materials Science: Materials in Electronics 30: 13604– 13616. DOI: 10.1007/s10854-019-01730-1.
- [27] A. Kulka, A. Milewska, W. Zaj ˛ac, K. Swierczek, E. ´ Hanc, and J. Molenda, (2012) “Possibility of modification of phosphoolivine by substitution in Li sublattice" Solid State Ionics 225: 575–579. DOI: 10.1016/j.ssi.2012.02.027.
- [28] D. Yi, X. Cui, N. Li, L. Zhang, and D. Yang, (2020) “Enhancement of electrochemical performance of LiFePO4@ C by Ga coating" ACS omega 5(17): 9752–9758. DOI: 10.1021/acsomega.9b04165.
- [29] Q. Zhang, S.-Z. Huang, J. Jin, J. Liu, Y. Li, H.-E. Wang, L.-H. Chen, B.-J. Wang, and B.-L. Su, (2016) “Engineering 3D bicontinuous hierarchically macro-mesoporous LiFePO4/C nanocomposite for lithium storage with high rate capability and long cycle stability" Scientific reports 6(1): 25942. DOI: 10.1038/srep25942.
- [30] H.-J. Kim, G.-H. Bae, S.-M. Lee, J.-H. Ahn, and J.-K. Kim, (2019) “Properties of lithium iron phosphate prepared by biomass-derived carbon coating for flexible lithium ion batteries" Electrochimica Acta 300: 18–25. DOI: 10.1016/j.electacta.2019.01.057.
- [31] X. Huang, Y. Du, P. Qu, F. Liang, Y. Dai, and Y. Yao, (2017) “Effect of carbon coating on the properties and electrochemical performance of LiFePO4/C composites by vacuum decomposition method" Int J Electrochem Sci 12: 7183–7196. DOI: 10.20964/2017.08.77.
- [32] R. Liu, J. Chen, Z. Li, Q. Ding, X. An, Y. Pan, Z. Zheng, M. Yang, and D. Fu, (2018) “Preparation of LiFePO4/C cathode materials via a green synthesis route for lithiumion battery applications" Materials 11(11): 2251. DOI: 10.3390/ma11112251.
- [33] J. A. Österreicher, C. Simson, A. Großalber, S. Frank, and S. Gneiger, (2021) “Spatial lithium quantification by backscattered electron microscopy coupled with energydispersive X-ray spectroscopy" Scripta Materialia 194: 113664. DOI: 10.1016/j.scriptamat.2020.113664.
- [34] P. Hovington, V. Timoshevskii, S. Burgess, H. Demers, P. Statham, R. Gauvin, and K. Zaghib, (2016) “Can we detect Li K x-ray in lithium compounds using energy dispersive spectroscopy?" Scanning 38(6): 571–578. DOI: 10.1002/sca.21302.
- [35] C. Li, Y. Xie, N. Zhang, L. Ai, Y. Liang, K. Tuo, X. Ye, G. Jia, and S. Li, (2019) “Optimization of LiFePO4 cathode material based on phosphorus doped graphite network structure for lithium ion batteries" Ionics 25: 927–937. DOI: 10.1007/s11581-018-2744-7.
- [36] G. Cárdenas, G. Cabrera, E. Taboada, and S. P. Miranda, (2004) “Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR" Journal of Applied Polymer Science 93(4): 1876–1885. DOI: 10.1002/app.20647.
- [37] H. Rasti, K. Parivar, J. Baharara, M. Iranshahi, and F. Namvar, (2017) “Chitin from the mollusc chiton: extraction, characterization and chitosan preparation" Iranian journal of pharmaceutical research: IJPR 16(1): 366.
- [38] A. F. Orliukas, K.-Z. Fung, V. Venckute, V. Ka- ˙ zlauskiene, J. Miškinis, A. Dindune, Z. Kanepe, J. Ro- ˙ nis, A. Maneikis, T. Šalkus, et al., (2014) “SEM/EDX, XPS, and impedance spectroscopy of LiFePO4 and LiFePO4/C ceramics" Lithuanian Journal of Physics 54(2): DOI: 10.3952/physics.v54i2.2919.
- [39] J. Menéndez, A. Arenillas, B. Fidalgo, Y. Fernández, L. Zubizarreta, E. G. Calvo, and J. M. Bermúdez, (2010) “Microwave heating processes involving carbon materials" Fuel Processing Technology 91(1): 1–8. DOI: 10.1016/j.fuproc.2009.08.021.
- [40] T. Kim, J. Lee, and K.-H. Lee, (2014) “Microwave heating of carbon-based solid materials" Carbon letters 15(1): 15–24. DOI: 10.5714/CL.2014.15.1.015.
- [41] G. Jiang and S. Xie, (2019) “Preparation and electrochemical properties of lignin porous carbon spheres as the negative electrode of lithium ion batteries" International Journal of Electrochemical Science 14(6): 5422–5434. DOI: 10.20964/2019.06.36.
- [42] E. Ratchai, M. Luengchavanon, K.-a. Techato, W. Limbuta, A. Chotikhun, and N. Y. Voo, (2023) “Characteristics of Carbon from Chitin-coated LiFePO4 and its Performance for Lithium Ion Battery" BioResources 18(3): 4399–4412. DOI: 10.15376/biores.18.3.4399-4412.
- [43] E. H. Mohan, V. Siddhartha, R. Gopalan, T. N. Rao, and D. Rangappa, (2014) “Urea and sucrose assisted combustion synthesis of LiFePO4/C nano-powder for lithium-ion battery cathode application" AIMS Materials Science 1(4): 191–201. DOI: 10.3934/matersci.2014.4.191.
- [44] J. Hagberg, H. A. Maples, K. S. Alvim, J. Xu, W. Johannisson, A. Bismarck, D. Zenkert, and G. Lindbergh, (2018) “Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries" Composites Science and Technology 162: 235–243. DOI: 10.1016/j.compscitech.2018.04.041.
- [45] H.-J. Kim, G.-H. Bae, S.-M. Lee, J.-H. Ahn, and J.-K. Kim, (2019) “Properties of lithium iron phosphate prepared by biomass-derived carbon coating for flexible lithium ion batteries" Electrochimica Acta 300: 18–25. DOI: 10.1016/j.electacta.2019.01.057.
- [46] X. Li, Y. Z. Jiang, X. K. Li, H. X. Jiang, J. L. Liu, J. Feng, S. B. Lin, and X. Guan, (2018) “Electrochemical property of LiFePO4/C composite cathode with different carbon sources" Rare Metals 37: 743–749. DOI: 10.1007/s12598-016-0781-9.
- [47] X. Ren, Z. Li, J. Cao, S. Tian, K. Zhang, J. Guo, L. Wen, and G. Liang, (2021) “Enhanced rate performance of the mortar-like LiFePO4/C composites combined with the evenly coated of carbon aerogel" Journal of Alloys and Compounds 867: 158776. DOI: 10.1016/j.jallcom.2021.158776.
- [48] M. T. Thi, C. Kim, S. Kwon, H. Kang, J. M. Ko, J. Kim, and W. Choi, (2023) “Investigation of the Properties of Anode Electrodes for Lithium–Ion Batteries Manufactured Using Cu, and Si-Coated Carbon Nanowall Materials" Energies 16(4): 1935. DOI: 10.3390/en16041935.
- [49] H. Yang, Y. Qiu, and X. Guo, (2015) “Prediction of state-of-health for nickel-metal hydride batteries by a curve model based on charge-discharge tests" Energies 8(11): 12474–12487. DOI: 10.3390/en81112322.
- [50] Y.-H. Chiang, W.-Y. Sean, and J.-C. Ke, (2011) “Online estimation of internal resistance and open-circuit voltage of lithium-ion batteries in electric vehicles" Journal of Power Sources 196(8): 3921–3932. DOI: 10.1016/j.jpowsour.2011.01.005.
- [51] Y. Wu and R. Holze, (2021) “Self-discharge in supercapacitors: Causes, effects and therapies: An overview" Electrochemical Energy Technology 7(1): 1–37. DOI: 10.1515/eetech-2020-0100.
- [52] J. Guo, Y. Li, K. Pedersen, and D.-I. Stroe, (2021) “Lithium-ion battery operation, degradation, and aging mechanism in electric vehicles: an overview" Energies 14(17): 5220. DOI: 10.3390/en14175220.
- [53] L. Zhang, Y. Qin, Y. Liu, Q. Liu, Y. Ren, A. N. Jansen, and W. Lu, (2018) “Capacity fading mechanism and improvement of cycling stability of the SiO anode for lithiumion batteries" Journal of The Electrochemical Society 165(10): A2102. DOI: 10.1149/2.0431810jes.
- [54] C. Ania, J. Parra, J. Menéndez, and J. Pis, (2007) “Microwave-assisted regeneration of activated carbons loaded with pharmaceuticals" Water research 41(15): 3299–3306. DOI: 10.1016/j.watres.2007.05.006.
- [55] N. Yesibolati, N. Umirov, A. Koishybay, M. Omarova, I. Kurmanbayeva, Y. Zhang, Y. Zhao, and Z. Bakenov, (2015) “High performance Zn/LiFePO4 aqueous rechargeable battery for large scale applications" Electrochimica Acta 152: 505–511. DOI: 10.1016/j.electacta.2014.11.168.
- [56] T.-J. Kuo, (2019) “Development of a comprehensive model for the coulombic efficiency and capacity fade of LiFePO4 batteries under different aging conditions" Applied Sciences 9(21): 4572. DOI: 10.3390/app9214572.
- [57] M. A. Marwat, M. Yasar, W. Ma, P. Fan, K. Liu, D. Lu, Y. Tian, C. Samart, B. Ye, and H. Zhang, (2020) “Significant energy density of discharge and charge–discharge efficiency in Ag@ BNN nanofillers-modified heterogeneous sandwich structure nanocomposites" ACS Applied Energy Materials 3(7): 6591–6601. DOI: 10.1021/acsaem.0c00770.
- [58] Y. Gao, Z. Pan, J. Sun, Z. Liu, and J. Wang, (2022) “High-energy batteries: beyond lithium-ion and their long road to commercialisation" Nano-micro letters 14(1): 94. DOI: 10.1007/s40820-022-00844-2.