Chia-Hua Lee1 and Wen-Chang Chen 1,2 1Department of Chemical Engineering National Taiwan University Taipei, Taiwan 106, R.O.C.
2Institute of Polymer Science and Engineering National Taiwan University Taipei, Taiwan 106, R.O.C.
Received:
February 14, 2003
Accepted:
April 21, 2003
Publication Date:
June 1, 2003
Download Citation:
||https://doi.org/10.6180/jase.2003.6.2.02
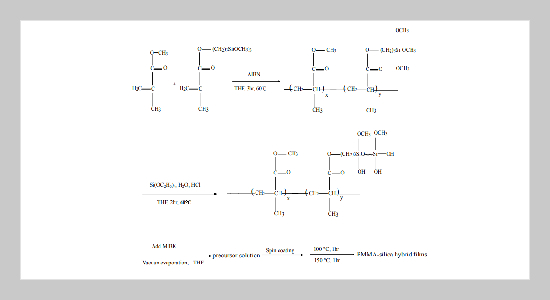
In this study, trialkoxycapped poly(methyl methacrylate) (PMMA)-silica hybrid films were successfully prepared for optical applications. The acrylic copolymer precursor with alkoxy side group was prepared using 2,2’-azobisisobutyronitrile (AIBN) as the initiator first. Then, it was reacted with partially hydrolyzed tetraethoxysilane solution, followed by spin coating and curing to obtain the thin films. The prepared hybrid films had high surface planarity and homogeneity. The optical properties of the prepared hybrid films could be tuned by the silica moiety. The refractive indices of the prepared films reduced from 1.495 to 1.460 by increasing the silica content. The near infrared optical spectra of the prepared films were shifted by the silica moiety. It was probably due to increase the anharmonicity of C-H bond by the silica moiety. The optical loss of the prepared optical waveguides was reduced from 0.243 to 0.198 dB/cm by increasing the silica content, which was less than that of the PMMA based waveguide. The trend on the optical loss was probably due to the reduction of the C-H number density and enhancing the anharmoncity of the C-H bond. The hybrid films prepared from the initiator of AIBN had a higher surface planarity than that from BPO and resulted in a lower optical loss of the prepared waveguides.ABSTRACT
Keywords:
Hybrid Materials, Thin Films, Optical Properties, Waveguide
REFERENCES