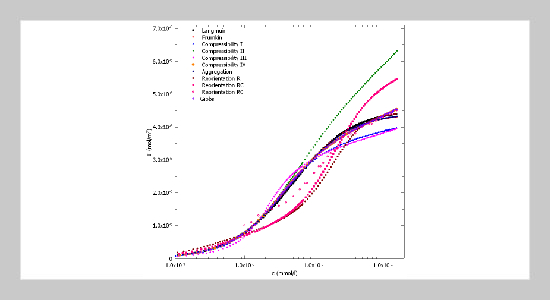
- [1] I. Rivas, A. J. Castellanos-Suárez, M. García-Sucre, E. Lopez, S. D. Rosales-Anzola, and G. Urbina-Villalba. “Surface dilational viscoelasticity of surfactants”. In: Topics in the Colloidal Aggregation and Interfacial Phe�nomena. Ed. by M. García-Sucre, A. J. Castellanos�Suarez, and J. Toro-Mendoza. Research Signpost, 2012, 201–223.
- [2] Z. Briceño-Ahumada and D. Langevin, (2017) “On the influence of surfactant on the coarsening of aqueous foams" Advances in Colloid and Interface Science 244: 124–131. DOI: https: //doi.org/10.1016/j.cis.2015.11.005.
- [3] J. Lucassen and M. Van Den Tempel, (1972) “Dy�namic measurements of dilational properties of a liquid interface" Chemical Engineering Science 27(6): 1283– 1291. DOI: https: //doi.org/10.1016/0009-2509(72)80104-0.
- [4] Y. Jayalakshmi, L. Ozanne, and D. Langevin, (1995) “Viscoelasticity of Surfactant Monolayers" Journal of Colloid and Interface Science 170(2): 358–366. DOI: https://doi.org/10.1006/jcis.1995.1113.
- [5] C. Stubenrauch and R. Miller, (2004) “Stability of Foam Films and Surface Rheology: An Oscillating Bubble Study at Low Frequencies" The Journal of Physical Chem�istry B 108(20): 6412–6421. DOI: 10.1021/jp049694e.
- [6] E. Santini, F. Ravera, M. Ferrari, C. Stubenrauch, A. Makievski, and J. Krägel, (2007) “A surface rheolog�ical study of non-ionic surfactants at the water–air in�terface and the stability of the corresponding thin foam films" Colloids and Surfaces A: Physicochemical and Engineering Aspects 298(1): 12–21. DOI: https: //doi.org/10.1016/j.colsurfa.2006.12.004.
- [7] E. Lucassen-Reynders, A. Cagna, and J. Lucassen, (2001) “Gibbs elasticity, surface dilational modulus and diffusional relaxation in nonionic surfactant monolayers" Colloids and Surfaces A: Physicochemical and En�gineering Aspects 186(1): 63–72. DOI: https: //doi.org/10.1016/S0927-7757(01)00483-6.
- [8] E. Aksenenko. “7. Software tools to interpret the ther�modynamics and kinetics of surfactant adsorption”. In: Surfactants. Ed. by V. Fainerman, D. Möbius, and R. Miller. 13. Studies in Interface Science. Elsevier, 2001, 619–648. DOI: https: //doi.org/10.1016/S1383-7303(01)80068-5.
- [9] D. Grigoriev and C. Stubenrauch, (2007) “Sur�face elasticities of aqueous β − dodecyl − D − maltosidesolutions : Acapillarywavestudy” Col�loids and Surfaces A: Physicochemical and Engineering Aspects 296(1): 67–75. DOI: https: //doi.org/10.1016/j.colsurfa.2006.09.025.
- [10] J. Boos, N. Preisig, and C. Stubenrauch, (2013) “Dila�tional surface rheology studies of n-dodecyl-β-d-maltoside, hexaoxyethylene dodecyl ether, and their 1:1 mixture" Advances in Colloid and Interface Science 197-198: 108–117. DOI: 10.1016/j.cis.2013.05.001.
- [11] W. Xiang, B. Tardy, L. Bai, C. Stubenrauch, and O. J. Rojas. “Chapter 12 - Measuring the Interfacial Be�havior of Sugar-Based Surfactants to Link Molecular Structure and Uses”. In: Biobased Surfactants (Second Edition). Ed. by D. G. Hayes, D. K. Solaiman, and R. D. Ashby. Second Edition. AOCS Press, 2019, 387–412. DOI: 10.1016/B978-0-12-812705-6.00012-5.
- [12] V. Fainerman, E. Lucassen-Reynders, and R. Miller, (1998) “Adsorption of surfactants and proteins at fluid interfaces" Colloids and Surfaces A: Physicochem�ical and Engineering Aspects 143(2): 141–165. DOI: https: //doi.org/10.1016/S0927-7757(98)00585-8.
- [13] V. Fainerman and R. Miller. “2. Thermodynamics of adsorption of surfactants at the fluid interfaces”. In: Surfactants. Ed. by V. Fainerman, D. Möbius, and R. Miller. 13. Studies in Interface Science. Elsevier, 2001, 99–188. DOI: https: //doi.org/10.1016/S1383-7303(01)80063-6.
- [14] V. Fainerman, R. Miller, E. Aksenenko, and A. Makievski. “3. Equilibrium adsorption properties of single and mixed surfactant solutions”. In: Surfac�tants. Ed. by V. Fainerman, D. Möbius, and R. Miller. 13. Studies in Interface Science. Elsevier, 2001, 189– 285. DOI: https: //doi.org/10.1016/S1383-7303(01)80064-8.
- [15] A. Frumkin, (1925) Zeitschrift für Physikalische Chemie 116U(1): 466–484. DOI: doi:10.1515/zpch�1925-11629.
- [16] V. Fainerman, S. Zholob, J. Petkov, and R. Miller, (2008) “C14EO8 adsorption characteristics studied by drop and bubble profile tensiometry" Colloids and Sur�faces A: Physicochemical and Engineering Aspects 323(1): 56–62. DOI: https: //doi.org/10.1016/j.colsurfa.2007.09.019.
- [17] U. Gehlert and D. Vollhardt, (2002) “Molecular Pack�ing and Textures of 1-Stearylamine-rac-glycerol Mono�layers" Langmuir 18(3): 688–693. DOI: 10.1021/la010867q.
- [18] V. B. Fainerman, R. Miller, and V. I. Kovalchuk, (2002) “Influence of the Compressibility of Adsorbed Layers on the Surface Dilational Elasticity" Langmuir 18(20): 7748–7752. DOI: 10.1021/la020024e.
- [19] V. B. Fainerman, R. Miller, and V. I. Kovalchuk, (2003) “Influence of the Two-Dimensional Compressibility on the Surface Pressure Isotherm and Dilational Elasticity of Do�decyldimethylphosphine Oxide" The Journal of Phys�ical Chemistry B 107(25): 6119–6121. DOI: 10.1021/jp021876q.
- [20] V. B. Fainerman, V. I. Kovalchuk, E. V. Aksenenko, M. Michel, M. E. Leser, and R. Miller, (2004) “Models of Two-Dimensional Solution Assuming the Internal Com�pressibility of Adsorbed Molecules: A Comparative Anaysis" The Journal of Physical Chemistry B 108(36): 13700–13705. DOI: 10.1021/jp049120+.
- [21] V. B. Fainerman and R. Miller, (1996) “Surface Ten�sion Isotherms for Surfactant Adsorption Layers Includ�ing Surface Aggregation" Langmuir 12(25): 6011–6014. DOI: 10.1021/la960457f.
- [22] E. V. Aksenenko, V. B. Fainerman, and R. Miller, (1998) “Dynamics of Surfactant Adsorption from So�lution Considering Aggregation within the Adsorption Layer" The Journal of Physical Chemistry B 102(31): 6025–6028. DOI: 10.1021/jp980664j.
- [23] V. Fainerman, R. Miller, E. Aksenenko, A. Makievski, J. Krägel, G. Loglio, and L. Liggieri, (2000) “Effect of surfactant interfacial orientation/aggregation on adsorp�tion dynamics" Advances in Colloid and Interface Science 86(1): 83–101. DOI: https: //doi.org/10.1016/S0001-8686(00)00033-6.
- [24] V. B. Fainerman, R. Miller, R. Wüstneck, and A. V. Makievski, (1996) “Adsorption Isotherm and Surface Tension Equation for a Surfactant with Changing Par�tial Molar Area. 1. Ideal Surface Layer" The Journal of Physical Chemistry 100(18): 7669–7675. DOI: 10.1021/jp960148y.
- [25] V. B. Fainerman, R. Miller, and R. Wüstneck, (1997) “Adsorption Isotherm and Surface Tension Equation for a Surfactant with Changing Partial Molar Area. 2. Non�ideal Surface Layer" The Journal of Physical Chem�istry B 101(33): 6479–6483. DOI: 10.1021/jp970746s.
- [26] R. Miller, E. Aksenenko, and V. Fainerman, (2001) “The Elasticity of Adsorption Layers of Reorientable Sur�factants" Journal of Colloid and Interface Science 236(1): 35–40. DOI: https: //doi.org/10.1006/jcis.2000.7386.
- [27] S. D. Rosales-Anzola, M. García-Sucre, G. Urbina�Villalba, and E. Lopez. “Surface dilational viscoelas�ticity of surfactants”. In: Topics in the Colloidal Aggrega�tion and Interfacial Phenomena. Ed. by M. García-Sucre, A. J. Castellanos-Suarez, and J. Toro-Mendoza. Re�search Signpost, 2012, 201–223.
- [28] V. Fainerman, S. Lylyk, E. Aksenenko, A. Makievski, J. Petkov, J. Yorke, and R. Miller, (2009) “Adsorption layer characteristics of Triton surfactants: 1. Surface ten�sion and adsorption isotherms" Colloids and Surfaces A: Physicochemical and Engineering Aspects 334(1): 1–7. DOI: https: //doi.org/10.1016/j.colsurfa.2008.09.015.
- [29] R. Bois, I. Pezron, and A. Nesterenko, (2020) “Dy�namic interfacial properties of sugar-based surfactants: Experimental study and modeling" Colloid and Inter�face Science Communications 37: 100293. DOI: https: //doi.org/10.1016/j.colcom.2020.100293.
- [30] C. Stubenrauch, V. B. Fainerman, E. V. Aksenenko, and R. Miller, (2005) “Adsorption Behavior and Dila�tional Rheology of the Cationic Alkyl Trimethylammo�nium Bromides at the Water/Air Interface" The Jour�nal of Physical Chemistry B 109(4): 1505–1509. DOI: 10.1021/jp046525l.
- [31] V. I. Kovalchuk, E. V. Aksenenko, E. Schneck, and R. Miller, (2023) “Surfactant Adsorption Layers: Exper�iments and Modeling" Langmuir 39(10): 3537–3545. DOI: 10.1021/acs.langmuir.2c03511.
- [32] M. Vrânceanu, K. Winkler, H. Nirschl, and G. Le�neweit, (2007) “Surface rheology of monolayers of phos�pholipids and cholesterol measured with axisymmetric drop shape analysis" Colloids and Surfaces A: Physic�ochemical and Engineering Aspects 311(1): 140–153. DOI: https: //doi.org/10.1016/j.colsurfa.2007.06.008.
- [33] G. Loglio, P. Pandolfini, A. Makievski, and R. Miller, (2003) “Calibration parameters of the pendant drop ten�siometer: assessment of accuracy" Journal of Colloid and Interface Science 265(1): 161–165. DOI: https: //doi.org/10.1016/S0021-9797(03)00138-3.
- [34] G. Loglio, P. Pandolfini, R. Miller, A. Makievski, J. Krägel, and F. Ravera, (2004) “Oscillation of interfacial properties in liquid systems: assessment of harmonic dis�tortion" Phys. Chem. Chem. Phys. 6: 1375–1379. DOI: 10.1039/B314592C.
- [35] S. I. Karakashev and A. V. Nguyen, (2009) “The im�portance of aspect ratio in profile analysis tensiometry" Journal of Colloid and Interface Science 330(2): 501–504. DOI: https: //doi.org/10.1016/j.jcis.2008.11.034.