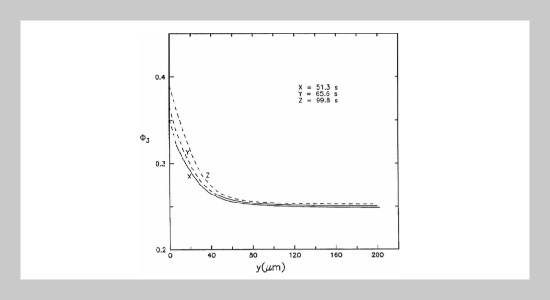
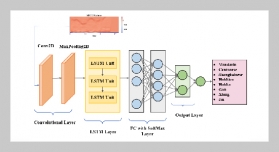
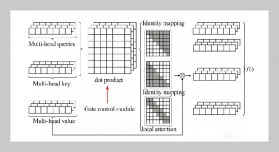
REFERENCES
- [1] Lin, D. J., Chang, C. L., Chen, T. C. and Cheng, L. P., “On the Structure of Porous Poly(Vinylidene Fluoride) Membrane Prepared by Phase Inversion from WaterNMP-PVDF System,” Tamkang Journal of Science and Engineering, Vol. 5, pp. 95�98 (2002).
- [2] Young, T. H., Chang, H. H., Lin, D. J. and Cheng, L. P., “Surface Modification of Microporous PVDF Membranes for Neuron Culture,” Journal of Membrane Science, Vol. 350, pp. 32�41 (2010).
- [3] Shih, C. H., Gryte, C. C. and Cheng, L. P., “Morphology of Membranes Formed by Isothermal Precipitation of Polyamide Solutions from Water/Formic Acid Systems,” Journal of Applied Polymer Science, Vol. 96, pp. 944�960 (2005).
- [4] Young, T. H., Lu, J. N., Lin, D. J., Chang, C. L., Chang, H. H. and Cheng, L. P., “Immobilization of l-Lysine on Dense and Porous Poly(Vinylidene Fluoride) Surfaces for Neuron Culture,” Desalination, Vol. 234, pp. 131� 143 (2008).
- [5] Lin, K. Y., Wang, D. M. and Lai, J. Y., “NonsolventInduced Gelation and Its Effect on Membrane Morphology,” Macromolecules, Vol. 35, pp. 6697�6706 (2002).
- [6] Zhao, Y. H., Zhu, B. K., Ma, X. T. and Xu, Y. Y., “Porous Membranes Modified by Hyperbranched Polymers: I. Preparation and Characterization of PVDF Membrane Using Hyperbranched Polyglycerol as Additive,” Journal of Membrane Science, Vol. 290, pp. 222�229 (2007).
- [7] Wang. X., Zhang, L., Sun, D., An, Q. and Chen, H., “Effect of Coagulation Bath Temperature on Formation Mechanism of Poly(Vinylidene Fluoride) Membrane,” Journal of Applied Polymer Science, Vol. 110, pp. 1656�1663 (2008).
- [8] Yeow, L. M., Liu, Y. T. and Li, K. “Morphological Study of Poly(Vinylidene Fluoride) Asymmetric Membranes: Effects of the Solvent, Additive, and Dope Temperature,” Journal of Applied Polymer Science, Vol. 92, pp. 1782�1789 (2004).
- [9] Blanco, J. F., Sublet, J., Nguyen, Q. T. and Schaetzel, P., “Formation and Morphology Studies of Different Polysulfones-Based Membranes Made by Wet Phase Inversion Process,” Journal of Membrane Science, Vol. 283, pp. 27�37 (2006).
- [10] Khayet, M., Mengual, J. I. and Mastsuura, T., “Porous Hydrophobic/Hydrophilic Composite Membranes: Application in Desalination Using Direct Contact Membrane Distillation,” Journal of Membrane Science, Vol. 252, pp. 101�113 (2005).
- [11] Cheng, L. P., Dwan, A. H. and Gryte, C. C., “Membrane Formation by Isothermal Precipitation in Polyamide-Formic Acid-Water Systems I. Description of Membrane Morphology,” Journal of Polymer Science, Polymer Physics, Vol. 33, pp. 211�222 (1995).
- [12] Cheng, L. P., Dwan, A. H. and Gryte, C. C., “Membrane Formation by Isothermal Precipitation in Polyamide-Formic Acid-Water Systems II. Precipitation Dynamics,” Journal of Polymer Science, Polymer Physics, Vol. 33, pp. 223�235 (1995).
- [13] Cohen, C., Tanny, G. B. and Prager, S., “DiffusionControlled Formation of Porous Structures in Ternary Polymer Systems,” Journal of Polymer Science, Polymer Physics, Vol. 17, pp. 477�489 (1979).
- [14] Reuvers, A. J., van den Berg, J. W. A. and Smolders, C. A., “Formation of Membranes by Means of Immersion Precipitation: Part I. A Model to Describe Mass Transfer during Immersion Precipitation,” Journal of Membrane Science, Vol. 34, pp. 45�65 (1987).
- [15] Reuvers, A. J. and Smolders, C. A., “Formation of Membranes by Means of Immersion Precipitation: Part II. the Mechanism of Formation of Membranes Prepared from the System Cellulose Acetate-AcetoneWater,” Journal of Membrane Science, Vol. 34, pp. 67�86 (1987).
- [16] Tsay, C. S. and McHugh, A. J., “Mass Transfer Modeling of Asymmetric Membrane Formation by Phase Inversion,” Journal of Polymer Science, Polymer Physics, Vol. 28, pp. 1327�1365 (1990).
- [17] Cheng, L. P., Soh, Y. S., Dwan, A. H. and Gryte, C. C., “An Improved Model for Mass Transfer during the Formation of Polymeric Membranes by the Immersion-Precipitation Process,” Journal of Polymer Science, Polymer Physics, Vol. 32, pp. 1413�1425 (1994).
- [18] Cheng, L. P., Dwan, A. H. and Gryte, C. C., “Measurements of Mutual Diffusivities in Concentrated Solutions of Membrane-Forming Polyamides and Cellulose Acetate,” Journal of Applied Polymer Science, Vol. 57, pp. 563�572 (1995).
- [19] Marinaccio, P. J. and Knight, R. A., U.S. Patent 3,876,738 (1975).
- [20] Pall, D. B., U. S. Patent 4,340,479 (1982).
- [21] Cheng, L. P., Dwan, A. H. and Gryte, C. C., “Isothermal Phase Behavior of Nylon-6, -66, and -610 Polyamides in Formic Acid-Water Systems,” Journal of Polymer Science, Polymer Physics, Vol. 32, pp. 1183� 1190 (1994).
- [22] Duda, J. L., Vrentas, J. S., Ju, S. T. and Liu, H. T., “Prediction of Diffusion Coefficients for Polymer-Solvent Systems,” AIChE Journal, Vol. 28, pp. 279�285 (1982).
- [23] Haase, R., Thermodynamics of Irreversible Process, Addison-Wesley, MA (1969).
- [24] Fitts, D. D., Nonequilibrium Thermodynamics, McGraw-Hill, New York (1962).
- [25] Cheng, L. P., Mechanism of Microporous Membrane Formation by Precipitation of Semicrystalline Polymers, Ph. D. Dissertation, Columbia University, New York, U.S.A. (1993).
- [26] Dunlop, P. J., “Frictional Coefficients for Binary and Ternary Isothermal Diffusion,” Journal of Physical Chemistry, Vol. 68, pp. 26�30 (1964).