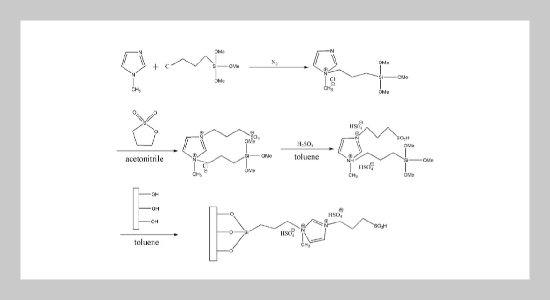
REFERENCES
- [1] Raji, F. and Pakizeh, M., “Study of Hg(II) Species Removal from Aqueous Solution Using Hybrid ZnCl2- MCM-41 Adsorbent,” Applied Surface Science, Vol. 282, pp. 415424 (2013). doi: 10.1016/j.apsusc.2013.05.145
- [2] Visa, M., Isac, L. and Duta, A., “Fly Ash Adsorbents for Multi-Cation Wastewater Treatment,” Applied Surface Science, Vol. 258, pp. 63456352 (2012). doi: 10. 1016/j.apsusc.2012.03.035
- [3] Kurczewska, J., Schroeder, G. and Narkiewicz, U., “Adsorption of Metal Ions on Magnetic Carbon Nanomaterials Bearing Chitosan-Functionalized Silica,” International Journal of Materials Research, Vol. 101, No. 12, pp. 15431547 (2010). doi: 10.3139/146. 110437
- [4] Correa, F. G. and Reyes, M. J., “Equilibrium and Thermodynamic Studies on the Adsorption of Eu(III) by Eggshell from Aqueous Solutions,” Adsorption Science & Technology, Vol. 31, No. 10, pp. 891902 (2013). doi: 10.1260/0263-6174.31.10.891
- [5] Shi, J., Feng, B., Lu, X. and Weng, J., “Adsorption of Bovine Serum Albumin onto Titanium Dioxide Nanotube Arrays,” International Journal of Materials Research, Vol. 103, No. 7, pp. 889896 (2012). doi: 10. 3139/146.110696
- [6] Fontanals, N., Ronka, S., Borrull, F., Trochimczuk, A. W. and Marce, R. M., “Supported Imidazolium Ionic Liquid Phases: A New Material for Solid-Phase Extraction,” Talanta, Vol. 80, No. 1, pp. 250256 (2009). doi: 10.1016/j.talanta.2009.06.068
- [7] Sun, J., Chen, Z. M., Ge, M. Y., Xu, L. and Zhai, M. L., “Selective Adsorption of Hg(II) by �-Radiation Synthesized Silica-Graft-Vinyl Imidazole Adsorbent,” Journal of Hazardous Materials, Vol. 244245, pp. 94101 (2013). doi: 10.1016/j.jhazmat.2012.11.043
- [8] Mohan, D. and Pittman Jr., C. U., “Activated Carbons and Low Cost Adsorbents for Remediation of Triand Hexavalent Chromium from Water,” Journal of Hazardous Materials, Vol. 137, No. 2, pp. 762811 (2006). doi: 10.1016/j.jhazmat.2006.06.060
- [9] Yadav, S., Srivastava, V., Banerjee, S., Weng, C. H. and Sharma, Y. C., “Adsorption Characteristics of Modified Sand for the Removal of Hexavalent Chromium Ions from Aqueous Solutions: Kinetic, Thermodynamic and Equilibrium Studies,” Catena, Vol. 100, pp. 120127 (2013). doi: 10.1016/j.catena.2012.08.002
- [10] Zhang, Q., Luo, J. and Wei, Y. Y., “A Silica Gel Supported Dual Acidic Ionic Liquid: an Efficient and Recyclable Heterogeneous Catalyst for the One-Pot Synthesis of Amidoalkyl Naphthols,” Green Chemistry, Vol. 12, No. 12, pp. 22462254 (2010). doi: 10.1039/ c0gc00472c
- [11] Liu, J. B., Yao, S., Wang, L.T., Zhu, W. X., Xu, J. and Song, H., “Adsorption of Bromophenol Blue from Aqueous Samples by Novel Supported Ionic Liquids,” Journal of Chemical Technology and Biotechnology, Vol. 89, No. 2, pp. 230238 (2014). doi: 10.1002/ jctb.4106
- [12] Albadarin, A. B., Mangwandi, C., Al-Muhtaseb, A., Walker, G. M., Allen, S. J. and Ahmad, M. N. M., “Kinetic and Thermodynamics of Chromium Ions Adsorption onto Low-Cost Dolomite Adsorbent,” Chemical Engineering Journal, Vol. 179, pp. 193202 (2012). doi: 10.1016/j.cej.2011.10.080
- [13] Panda, L., Das, B., Rao, D. S. and Mishra, B. K., “Application of Dolochar in the Removal of Cadmium and Hexavalent Chromium Ions from Aqueous Solutions,” Journal of Hazardous Materials, Vol. 192, No. 2, pp. 822831 (2011). doi: 10.1016/j.jhazmat.2011.05.098
- [14] Venditti, F., Cuomo, F., Ceglie, A., Ambrosone, L. and Lopez, F., “Effects of Sulfate Ions and Slightly Acidic pH Conditions on Cr(VI) Adsorption onto Silica Gelatin Composite,” Journal of Hazardous Materials, Vol. 173, No. 13, pp. 552557 (2010). doi: 10.1016/j. jhazmat.2009.08.121
- [15] Jia, Z., Wang, Q., Ren, D. and Zhu, R., “Fabrication of One-Dimensional Mesoporous Alpha-Fe2O3 Nanostructure Via Self-Sacrificial Template and its Enhanced Cr(VI) Adsorption Capacity,” Applied Surface Science, Vol. 264, pp. 255260 (2013). doi: 10.1016/j. apsusc.2012.09.179
- [16] Li, W., Tang, Y., Zeng, Y. T., Tong, Z. F., Liang, D. and Cui, W. W., “Adsorption Behavior of Cr(VI) Ions on Tannin-Immobilized Activated Clay,” Chemical Engineering Journal, Vol. 193194, pp. 8895 (2012). doi: 10.1016/j.cej.2012.03.084
- [17] Wu, Y., Luo, H., Wang, H., Wang, C., Zhang, J. and Zhang, Z., “Adsorption of Hexavalent Chromium from Aqueous Solutions by Graphene Modified with Cetyltrimethylammonium Bromide,” Journal of Colloid and Interface Science, Vol. 394, pp. 183191 (2013). doi: 10.1016/j.jcis.2012.11.049
- [18] Jung, C., Heo, J., Han, J., Her, N. and Lee, S. J., “Hexavalent Chromium Removal by Various Adsorbents: Powdered Activated Carbon, Chitosan and Single/MultiWalled Carbon Nanotubes,” Seperation and Purification Technology, Vol. 106, pp. 6371 (2013). doi: 10.1016/j.seppur.2012.12.028
- [19] Wang, X. S., Chen, L. F., Li, F. Y., Chen, K. L., Wan, W. Y. and Tang, Y. J., “Removal of Cr(VI) with WheatResidue Derived Black Carbon: Reaction Mechanism and Adsorption Performance,” Journal of Hazardous Material, Vol. 175, No. 13, pp. 816822 (2010). doi: 10.1016/j.jhazmat.2009.10.082
- [20] Gupta, V. K., Rastogi, A. and Nayak, A., “Adsorption Studies on the Removal of Hexavalent Chromium from Aqueous Solution Using a Low Cost Fertilizer Industry Waste Material,” Journal of Colloid and Interface Science, Vol. 342, No. 1, pp. 135141 (2010). doi: 10.1016/j.jcis.2009.09.065