Shucong Zhen This email address is being protected from spambots. You need JavaScript enabled to view it.1, Xiaohui Dong1, Gloria Appiah-Sefah2, Mei Pan1 and Dekun Zhou1 1School of Architecture and Construction, Yancheng Institute of Technology, Yancheng 224051, P.R. China
2College of Environment, Hohai University, Nanjing 210009, P.R. China
Received:
May 5, 2014
Accepted:
September 29, 2014
Publication Date:
December 1, 2014
Download Citation:
||https://doi.org/10.6180/jase.2014.17.4.08
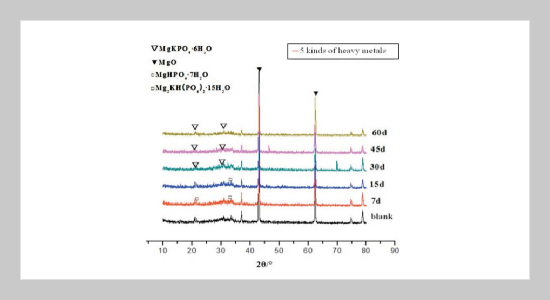
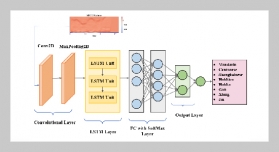
To study the changes in hydration products over time during the solidification/stabilization process of magnesium potassium phosphate cementing material (MKPC) and to reveal the solidification mechanism of heavy metal elements in MKPC, methods, such as scanning electron microscope-energy spectrum (SEM-EDS), X ray diffraction (XRD), Fourier Transform Infrared Spectroscopy (FTIR) etc., were used to analyze the composition and microstructure of MKPC solidified body products with different production time, which are further adulterated with heavy metals, such as Zn, Cu, Ni, Cd and Cr, as well as changes in phases of hydration products. The results showed that the preliminary, intermediate and the final hydration products were MgHPO47H2O, Mg2KH(PO4)215H2O and MgKPO46H2O(MKP), respectively on day 7, day 15, day 30, day 45 and day 60 after the curing of the solidified body, which was adulterated with heavy metals. Based on the analysis of the result of Cu2+ solidification test through FTIR, Mg2+ was replaced by Cu2+ in MKPC hydration products to generate CuKPO4, and the structure of the original hydration products did not have a crystal lattice change. The scanning electron microscope image (SEM) showed that cracks and harmful voids in the MKPC solidified body decreased gradually during the curing period between day 7 and day 60, and the effect of heavy metal solidification was strengthened. Based on energy spectrum analysis, heavy metal ions existing in the hydration products and MKPC could be used to solidify heavy metals. It was thus concluded that MKPC could solidify heavy metals, primarily due to the fact that heavy metals are capable of replacing Mg2+. Also, they participate in a variety of chemical reactions to generate heavy metal phosphate, which could react with heavy metal ions and precipitate them. Such sediments cement and the cemented body can seal partial heavy metal ions, and the combined actions could further solidify and stabilize heavy metals.ABSTRACT
Keywords:
Magnesium Potassium Phosphate Cementing (MKPC), Heavy Metals, Solidification/ Stabilization, Hydration Products
REFERENCES