Chein-Ho Huang This email address is being protected from spambots. You need JavaScript enabled to view it.1, Wen-Yung Shu2 , Hsien-Ming Wu2 , Hsiou-Jeng Shy2 and Shirley C. Wei3 1Chemistry Department, Soochow University, Taipei, Taiwan 111, ROC
2Materials & Electro-Optics Research Division of Chung-Shan Institute of Science and Technology, Lung-Tan, Taiwan, ROC
3Poliyen Company, Hershey, PA, USA
Received:
June 20, 2007
Accepted:
April 28, 2008
Publication Date:
December 1, 2008
Download Citation:
||https://doi.org/10.6180/jase.2008.11.4.01
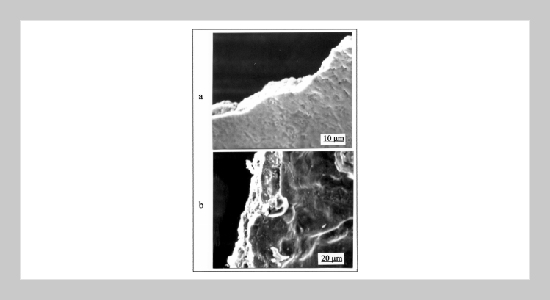
The conductivity of manganese dioxide obtained through pyrolysis of manganese nitrate solution was enhanced by the presence of ammonium nitrate. During the pyrolysis process ammonium nitrate generated oxidizing gases which reduced the amount of Mn(II) and Mn(III) (manganese sesquioxide) homogeneously in the pyrolytic product. Thus, the purity and the composition uniformity of the pyrolytic product were improved. Moreover, the BET surface area of the pyrolytic products obtained from most manganese solutions also decreased by the presence of ammonium nitrate. Consequently, pyrolytic manganese dioxide having high conductivity and solid tantalum capacitor exhibiting better characteristics were obtained.ABSTRACT
Keywords:
Conductivity, Manganese Dioxide, Pyrolysis
REFERENCES