Chung-Hsin Lu This email address is being protected from spambots. You need JavaScript enabled to view it.1, Hsuan-Hao Chang1 and Chi Yen1 1Electronic and Electro-Optical Ceramics Laboratory Department of Chemical Engineering National Taiwan University Taipei, Taiwan 106, R.O.C
Received:
August 17, 2004
Accepted:
October 1, 2004
Publication Date:
December 1, 2004
Download Citation:
||https://doi.org/10.6180/jase.2004.7.4.01
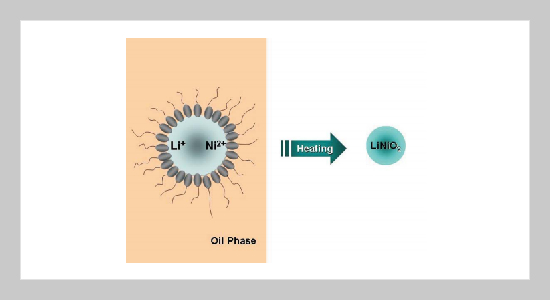
Lithium nickel oxide (LiNiO2) powders with an R3 m structure are successfully synthesized via a developed reverse microemulsion process. This method can effectively reduce the calcination temperature for synthesizing monophasic LiNiO2 powders to 700 ºC, which is much lower than that in the solid-state process. Nanosized LiNiO2 powders with narrow particle size distribution are obtained via the microemulsion process. Heating the precursors in pure oxygen results in the formation of LiNiO2 powders with an orderly arranged structure. The electrochemical analysis reveals that the microemulsion-derived LiNiO2 powders exhibit high discharge capacity and good cycleability.ABSTRACT
Keywords:
Electroceramics, Microemulsion, Lithium-ion Batteries, Powder Technology
REFERENCES