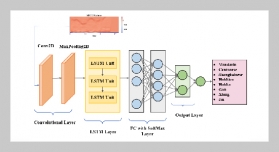
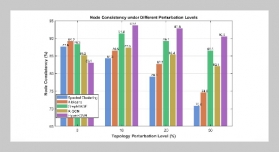
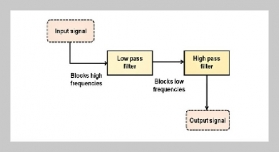
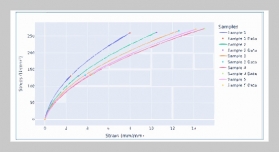
S. P. S. N Buddhika Sampath Kumara1,2,3, S. W. M. A. Ishantha Senevirathne1,3, Asha Mathew1,3,6, Preetha Ebenezer1,3, Tejasri Yarlagadda5, Laura Bray1,3, Mohammad Mirkhalaf1,3,4This email address is being protected from spambots. You need JavaScript enabled to view it., and Prasad K. D. V. Yarlagadda1,2,3,6This email address is being protected from spambots. You need JavaScript enabled to view it.
1School of Mechanical, Medical and Process Engineering, Faculty of Engineering, Queensland University of Technology (QUT), Brisbane, QLD, Australia
2Australian Research Council Training Centre for Multiscale 3D Imaging, Modelling, and Manufacturing, Queensland University of Technology (QUT), Brisbane, QLD, Australia
3Centre for Biomedical Technologies, Queensland University of Technology (QUT), Brisbane, QLD, Australia
4Centre for Materials Science, Queensland University of Technology (QUT), Brisbane, QLD, Australia
5Centre for Immunology and Infection Control, Queensland University of Technology (QUT), Brisbane, QLD, Australia
6School of Engineering, University of Southern Queensland, Springfield, QLD, Australia
- [1] A. Abdulkareem, P. Kasak, M. G. Nassr, A. A. Mah�moud, M. K. A. Al-Ruweidi, K. J. Mohamoud, M. K. Hussein, and A. Popelka, (2021) “Surface modification of poly (lactic acid) film via cold plasma assisted grafting of fumaric and ascorbic acid" Polymers 13(21): 3717. DOI: 10.3390/polym13213717.
- [2] H. Al Abdallah, B. Abu-Jdayil, and M. Z. Iqbal, (2022) “The effect of alkaline treatment on poly (lactic acid)/date palm wood green composites for thermal insulation" Poly�mers 14(6): 1143. DOI: 10.3390/polym14061143.
- [3] T. Aoki, H. Izumi, and S. Aoyagi. “Fabrication of a mi�cro needle made of biodegradable polymer material”. In: Mechatronics for safety, security and dependability in a new era. Elsevier, 2007, 381–384. DOI: 10.1016/B978-008044963-0/50077-1.
- [4] S. Aoyagi, H. Izumi, Y. Isono, M. Fukuda, and H. Ogawa, (2007) “Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle" Sensors and Actuators A: Physical 139(1-2): 293–302. DOI: 10.1016/j.sna.2006.11.022.
- [5] F. Asghari, M. Samiei, K. Adibkia, A. Akbarzadeh, and S. Davaran, (2017) “Biodegradable and biocompati�ble polymers for tissue engineering application: a review" Artificial cells, nanomedicine, and biotechnology 45(2): 185–192. DOI: 10.3109/21691401.2016.1146731.
- [6] A. Bagno and C. Di Bello, (2004) “Surface treatments and roughness properties of Ti-based biomaterials" Jour�nal of materials science: materials in medicine 15(9): 935–949. DOI: 10.1023/B:JMSM.0000042679.28493.7f.
- [7] B. D. Boyan, V. L. Sylvia, Y. Liu, R. Sagun, D. L. Cochran, C. H. Lohmann, D. D. Dean, and Z. Schwartz, (1999) “Surface roughness mediates its ef�fects on osteoblasts via protein kinase A and phospho�lipase A2" Biomaterials 20(23-24): 2305–2310. DOI: 10.1016/S0142-9612(99)00159-3.
- [8] W. Chen, L. Nichols, F. Brinkley, K. Bohna, W. Tian, M. W. Priddy, and L. B. Priddy, (2021) “Alkali treat�ment facilitates functional nano-hydroxyapatite coating of 3D printed polylactic acid scaffolds" Materials Science and Engineering: C 120: 111686. DOI: 10.1016/j.msec.2020.111686.
- [9] Y. Cheng, X. Ma, T. Franklin, R. Yang, and C. I. Moraru, (2023) “Mechano-Bactericidal Surfaces: Mech�anisms, Nanofabrication, and Prospects for Food Appli�cations" Annual Review of Food Science and Tech�nology 14(1): 449–472. DOI: 10.1146/annurev-food�060721-022330.
- [10] B. W. Chieng, N. A. Ibrahim, W. M. Z. Wan Yunus, and M. Z. Hussein, (2013) “Poly (lactic acid)/poly (ethy�lene glycol) polymer nanocomposites: Effects of graphene nanoplatelets" Polymers 6(1): 93–104. DOI: 10.3390/polym6010093.
- [11] F. Ebrahimi and H. Ramezani Dana, (2022) “Poly lactic acid (PLA) polymers: from properties to biomedical appli�cations" International Journal of Polymeric Materi�als and Polymeric Biomaterials 71(15): 1117–1130. DOI: 10.1080/00914037.2021.1944140.
- [12] W. H. Hoidy, M. B. Ahmad, E. A. J. Al-Mulla, and N. A. B. Ibrahim, (2010) “Preparation and characteriza�tion of polylactic acid/polycaprolactone clay nanocompos�ites" J. Appl. Sci 10(2): 97–106. DOI: 10.3923/jas.2010.97.106.
- [13] G. Hazell, L. E. Fisher, W. A. Murray, A. H. Nobbs, and B. Su, (2018) “Bioinspired bactericidal surfaces with polymer nanocone arrays" Journal of colloid and in�terface science 528: 389–399. DOI: 10.1016/j.jcis.2018.05.096.
- [14] K. M. Hotchkiss, K. T. Sowers, and R. Olivares�Navarrete, (2019) “Novel in vitro comparative model of osteogenic and inflammatory cell response to dental implants" Dental Materials 35(1): 176–184. DOI: 10. 1016/j.dental.2018.11.011.
- [15] H. Ikram, A. Al Rashid, and M. Koç, (2022) “Synthe�sis and characterization of hematite (α-Fe2O3) reinforced polylactic acid (PLA) nanocomposites for biomedical appli�cations" Composites Part C: Open Access 9: 100331. DOI: 10.1016/j.jcomc.2022.100331.
- [16] R. Jiang, Y. Yi, L. Hao, Y. Chen, L. Tian, H. Dou, J. Zhao, W. Ming, and L. Ren, (2021) “Thermoresponsive nanostructures: from mechano-bactericidal action to bac�teria release" ACS Applied Materials & Interfaces 13(51): 60865–60877. DOI: 10.1021/acsami.1c16487.
- [17] A. Karthikeyan, M. Girard, M.-J. Dumont, G. Chouinard, and J. R. Tavares, (2022) “Surface modifi�cation of commercially available PLA polymer Mesh" In�dustrial & Engineering Chemistry Research 61(47): 17297–17305. DOI: 10.1021/acs.iecr.2c02502.
- [18] S. B. S. Kumara, S. A. I. Senevirathne, A. Mathew, L. Bray, M. Mirkhalaf, and P. K. Yarlagadda, (2023) “Progress in Nanostructured Mechano-Bactericidal Poly�meric Surfaces for Biomedical Applications" Nanomate�rials 13(20): 2799. DOI: 10.3390/nano13202799.
- [19] L.-T. Lim, R. Auras, and M. Rubino, (2008) “Pro�cessing technologies for poly (lactic acid)" Progress in polymer science 33(8): 820–852. DOI: 10.1016/j.progpolymsci.2008.05.004.
- [20] B. Majhy, P. Priyadarshini, and A. Sen, (2021) “Ef�fect of surface energy and roughness on cell adhesion and growth–facile surface modification for enhanced cell cul�ture" RSC advances 11(25): 15467–15476. DOI: 10.1039/D1RA02402G.
- [21] G. R. M. Matos, (2021) “Surface roughness of dental implant and osseointegration" Journal of maxillofacial and oral surgery 20: 1–4. DOI: 10.1007/s12663-020- 01437-5.
- [22] D. W. Mayo, F. A. Miller, and R. W. Hannah. Course notes on the interpretation of infrared and Raman spectra. John Wiley & Sons, 2004. DOI: 10.1002/0471690082.
- [23] J. P. Mofokeng, A. Luyt, T. Tábi, and J. Kovács, (2012) “Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices" Journal of Thermoplastic Composite Materials 25(8): 927–948. DOI: 10.1177/0892705711423291.
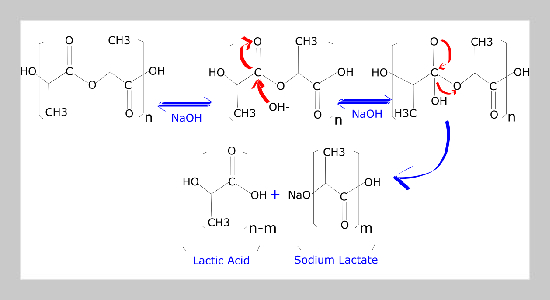
- [24] M. M. Sabee, N. Kamalaldin, B. Yahaya, and Z. A. Hamid, (2016) “Characterization and in vitro study of surface modified PLA microspheres treated with NaOH" Journal of Polymer Materials 33(1): 191. DOI: 10.32381/JPM.
- [25] A. B. D. Nandiyanto, R. Oktiani, and R. Ragadhita, (2019) “How to read and interpret FTIR spectroscope of organic material" Indonesian Journal of Science and Technology 4(1): 97–118. DOI: 10.17509/ijost.v4i1.15806.
- [26] E. Nasatzky, J. Gultchin, and Z. Schwartz, (2003) “The role of surface roughness in promoting osteointegration" Refu’at Ha-peh Veha-shinayim (1993) 20(3): 8–19.
- [27] D. Patil, V. Golia, M. Overland, M. Stoller, and K. Chatterjee, (2023) “Mechanobactericidal nanotopogra�phy on nitrile surfaces toward antimicrobial protective gear" ACS macro letters 12(2): 227–233. DOI: 10.1021/acsmacrolett.2c00697.
- [28] M. A. Pop, C. Croitoru, T. Bedo, V. Geaman, I. Radomir, M. Cosnita, S. M. Zaharia, L. A. Chicos, and I. Milosan, (2019) “Structural changes during 3D printing of bioderived and synthetic thermoplastic mate�rials" Journal of Applied Polymer Science 136(17): 47382. DOI: 10.1002/app.47382.
- [29] M. Schneider, N. Fritzsche, A. Puciul-Malinowska, A. Bali´s, A. Mostafa, I. Bald, S. Zapotoczny, and A. Taubert, (2020) “Surface etching of 3D printed poly (lac�tic acid) with NaOH: a systematic approach" Polymers 12(8): 1711. DOI: 10.3390/polym12081711.
- [30] C. Shasteen and Y. B. Choy, (2011) “Controlling degra�dation rate of poly (lactic acid) for its biomedical appli�cations" Biomedical Engineering Letters 1: 163–167. DOI: 10.1007/s13534-011-0025-8.
- [31] D. Da Silva, M. Kaduri, M. Poley, O. Adir, N. Krin�sky, J. Shainsky-Roitman, and A. Schroeder, (2018) “Biocompatibility, biodegradation and excretion of poly�lactic acid (PLA) in medical implants and theranostic systems" Chemical Engineering Journal 340: 9–14. DOI: 10.1016/j.cej.2018.01.010.
- [32] M. Singhvi, S. Zinjarde, and D. Gokhale, (2019) “Poly�lactic acid: synthesis and biomedical applications" Jour�nal of applied microbiology 127(6): 1612–1626. DOI: 10.1111/jam.14290.
- [33] P. Singla, R. Mehta, D. Berek, and S. Upadhyay, (2012) “Microwave assisted synthesis of poly (lactic acid) and its characterization using size exclusion chromatography" Journal of Macromolecular Science, Part A 49(11): 963–970. DOI: 10.1080/10601325.2012.722858.
- [34] C. Tham, Z. A. A. Hamid, Z. Ahmad, and H. Ismail, (2014) “Surface engineered poly (lactic acid)(PLA) mi�crospheres by chemical treatment for drug delivery sys�tem" Key Engineering Materials 594: 214–218. DOI: 10.4028/www.scientific.net/KEM.594-595.214.
- [35] R. Vaid, E. Yildirim, M. A. Pasquinelli, and M. W. King, (2021) “Hydrolytic degradation of polylactic acid fibers as a function of ph and exposure time" Molecules 26(24): 7554. DOI: 10.3390/molecules26247554.
- [36] Y. Deng, X. Liu, A. Xu, L. Wang, Z. Luo, Y. Zheng, F. Deng, J. Wei, Z. Tang, and S. Wei, (2015) “Effect of sur�face roughness on osteogenesis in vitro and osseointegra�tion in vivo of carbon fiber-reinforced polyetheretherketone– nanohydroxyapatite composite" International journal of nanomedicine: 1425–1447. DOI: 10.2147/IJN.S75557.
- [37] J. Xia, Y. Yuan, H. Wu, Y. Huang, and D. A. Weitz, (2020) “Decoupling the effects of nanopore size and sur�face roughness on the attachment, spreading and differen�tiation of bone marrow-derived stem cells" Biomaterials 248: 120014. DOI: 10.1016/j.biomaterials.2020.120014.
- [38] M. Zhao, L. Dai, N. Zhong, Z. Wang, M. Chen, B. Li, B. Luo, B. Tang, S. Shi, T. Song, et al., (2017) “Wet etching technique for fabrication of a high-quality plastic optical fiber sensor" Applied Optics 56(31): 8845–8850. DOI: 10.1364/AO.56.008845.
- [39] B. Tyler, D. Gullotti, A. Mangraviti, T. Utsuki, and H. Brem, (2016) “Polylactic acid (PLA) controlled delivery carriers for biomedical applications" Advanced drug delivery reviews 107: 163–175. DOI: 10.1016/j.addr.2016.06.018.