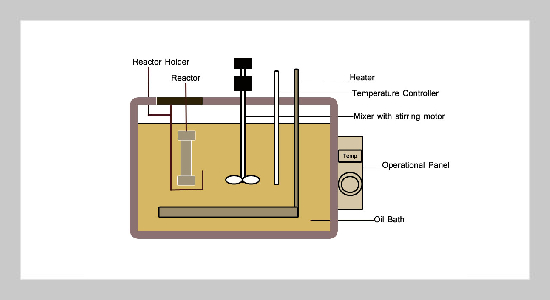
- [1] L. Du, P. J. Arauzo, M. F. Meza Zavala, Z. Cao, M. P. Olszewski, and A. Kruse, (2020) “Towards the proper�ties of different biomass-derived proteins via various ex�traction methods" Molecules 25(3): 488. DOI: 10.3390/molecules25030488.
- [2] S. Ibrahim, R. Santos, and S. Bowra, (2018) “Opti�mization of subcritical water mediated extraction of apple pomace polyphenolics and their antioxidant activity" Jour�nal of Chromatography & Separation Techniques 9(05):
- [3] X. Liang, Q. Fan, et al., (2013) “Application of sub�critical water extraction in pharmaceutical industry" Journal of Materials Science and Chemical Engi�neering 1(05): 1.
- [4] A. M. Silva, A. S. Luís, M. M. Moreira, R. Ferraz, T. Brezo-Borjan, J. Švarc-Gaji´c, P. C. Costa, C. Delerue�Matos, and F. Rodrigues, (2022) “Influence of tempera�ture on the subcritical water extraction of Actinidia arguta leaves: A screening of pro-healthy compounds" Sustain�able Chemistry and Pharmacy 25: 100593. DOI: 10.1016/j.scp.2021.100593.
- [5] M. Polikovsky, A. Gillis, E. Steinbruch, A. Robin, M. Epstein, A. Kribus, and A. Golberg, (2020) “Biorefin�ery for the co-production of protein, hydrochar and addi�tional co-products from a green seaweed Ulva sp. with subcritical water hydrolysis" Energy Conversion and Management 225: 113380. DOI: 10.1016/j.enconman.2020.113380.
- [6] M. N. Nasrabadi, A. S. Doost, and R. Mezzenga, (2021) “Modification approaches of plant-based proteins to improve their techno-functionality and use in food prod�ucts" Food Hydrocolloids 118: 106789. DOI: 10.1016/j.foodhyd.2021.106789.
- [7] K.-X. Zhu, X.-H. Sun, and H.-M. Zhou, (2009) “Op�timization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles" Journal of Ce�real Science 50(2): 266–271. DOI: 10.1016/j.jcs.2009.06.006.
- [8] C. Ferreira, M. M. Moreira, C. Delerue-Matos, and M. Sarraguça, (2023) “Subcritical Water Extraction to Valorize Grape Biomass—A Step Closer to Circular Economy" Molecules 28(22): 7538. DOI: 10.3390/molecules28227538.
- [9] S. Areeya, E. J. Panakkal, M. Sriariyanun, T. Kangsadan, A. Tawai, S. Amornraksa, U. W. Hart�ley, and P. Yasurin, (2023) “A review on chemical pre�treatment of lignocellulosic biomass for the production of bioproducts: mechanisms, challenges and applications" Applied Science and Engineering Progress 16(3): 6767–6767. DOI: 10.14416/j.asep.2023.02.008.
- [10] D. Jose, N. Kitiborwornkul, M. Sriariyanun, and K. Keerthi, (2022) “A review on chemical pretreatment methods of lignocellulosic biomass: Recent advances and progress" Applied Science and Engineering Progress 15(4): 6210–6210. DOI: 10.14416/j.asep.2022.08.001.
- [11] J. Zhang, C. Wen, H. Zhang, Y. Duan, and H. Ma, (2020) “Recent advances in the extraction of bioactive compounds with subcritical water: A review" Trends in Food Science & Technology 95: 183–195. DOI: 10.1016/j.tifs.2019.11.018.
- [12] N. L. Rahmah, S. M. M. Kamal, A. Sulaiman, F. S. Taip, S. I. Siajam, et al., (2022) “Optimization Of Pheno�lic Compounds And Antioxidant Extraction From Piper Betle Linn. Leaves Using Pressurized Hot Water" Jour�nal of Applied Science and Engineering 26(2): 175– 184. DOI: 10.6180/jase.202302_26(2).0003.
- [13] N. H. Zainan, M. A. M. Sapardi, B. C. H. Ho, S. I. Sia�jam, S. M. M. Kamal, M. K. Danquah, and R. Harun, (2022) “Correction to: Kinetic and thermodynamic char�acterization of amino acids generation via subcritical wa�ter reaction of microalgae Nannochloropsis sp. biomass" Biomass Conversion and Biorefinery: 1–1. DOI: 10.1007/s13399-021-01908-w.
- [14] W. Abdelmoez and H. Yoshida, (2013) “Production of amino and organic acids from protein using sub-critical water technology" International Journal of Chemical Reactor Engineering 11(1): 369–384. DOI: 10.1515/ijcre-2013-0017.
- [15] H. D. D. Ziero, L. C. Ampese, W. G. Sganzerla, P. C. Torres-Mayanga, M. T. Timko, S. I. Mussatto, and T. Forster-Carneiro, (2022) “Subcritical water hydrolysis of poultry feathers for amino acids production" The Journal of Supercritical Fluids 181: 105492. DOI: 10.1016/j.supflu.2021.105492.
- [16] Y. Cheng, F. Xue, S. Yu, S. Du, and Y. Yang, (2021) “Subcritical water extraction of natural products" Molecules 26(13): 4004.
- [17] F. He, (2011) “Bradford protein assay" Bio-protocol: e45–e45.
- [18] M.-J. Ko, M.-R. Kwon, and M.-S. Chung, (2020) “Pilot�scale subcritical-water extraction of nodakenin and de�cursin from Angelica gigas Nakai" Food Science and Biotechnology 29: 631–639.
- [19] A. A. Suleiman, U. A. Abdullahi, A. Suleiman, S. A. Suleiman, and H. U. Abubakar, (2022) “Correla�tion and regression model for physicochemical quality of groundwater in the Jaen District of Kano State, Nige�ria" Journal of Statistical Modeling & Analytics (JOSMA) 4(1):
- [20] V. V. Acharya and P. Chaudhuri, (2021) “Modalities of protein denaturation and nature of denaturants" Interna�tional Journal of Pharmaceutical Sciences Review and Research 69(2): 19–24.
- [21] G. Náthia-Neves and E. Alonso, (2024) “Optimiza�tion of the subcritical water treatment from sunflower by-product for producing protein and sugar extracts" Biomass Conversion and Biorefinery 14(2): 1637–1650.
- [22] A. H. Asl and M. Khajenoori, (2013) “Subcritical water extraction" Mass Transfer-Advances in sustainable energy and environment oriented numerical mod�eling: 459–487.
- [23] B. Díaz-Reinoso, S. Rivas, J. Rivas, and H. Domínguez, (2023) “Subcritical water extraction of es�sential oils and plant oils" Sustainable Chemistry and Pharmacy 36: 101332.
- [24] S. Joki´c, T. Gagi´c, Ž. Knez, D. Šubari´c, and M. Šk�erget, (2018) “Separation of active compounds from food by-product (cocoa shell) using subcritical water extraction" Molecules 23(6): 1408. DOI: 10.3390/molecules23061408.
- [25] T. Okuda, A. U. Baes, W. Nishijima, and M. Okada, (2001) “Coagulation mechanism of salt solution-extracted active component in Moringa oleifera seeds" Water re�search 35(3): 830–834. DOI: 10.1016/S0043-1354(00)00296-7.
- [26] V. V. Acharya and P. Chaudhuri, (2021) “Modalities of protein denaturation and nature of denaturants" Interna�tional Journal of Pharmaceutical Sciences Review and Research 69(2): 19–24.
- [27] S. Awaluddin, S. Thiruvenkadam, S. Izhar, Y. Hi�royuki, M. K. Danquah, R. Harun, et al., (2016) “Sub�critical water technology for enhanced extraction of bio�chemical compounds from Chlorella vulgaris" BioMed research international 2016: DOI: 10.1155/2016/5816974.
- [28] J. Lu, X. Feng, Y. Han, and C. Xue, (2014) “Opti�mization of subcritical fluid extraction of carotenoids and chlorophyll a from Laminaria japonica Aresch by response surface methodology" Journal of the Science of Food and Agriculture 94(1): 139–145. DOI: 10.1002/jsfa.6224.
- [29] M. Choudhary and S. Neogi, (2017) “A natural coagu�lant protein from Moringa oleifera: isolation, characteri�zation, and potential use for water treatment" Materials Research Express 4(10): 105502. DOI: 10.1088/2053-1591/aa8b8c.
- [30] A. N. Jones and J. Bridgeman, (2019) “Identifying Molecular Mass of Coagulant Protein from Edible Hi�biscus Seeds Using SDS-PAGE Analysis" Journal of Environmental Engineering 145(11): 04019077. DOI: 10.1061/(ASCE)EE.1943-7870.0001595.
- [31] N. A. Awang and H. A. Aziz, (2012) “Hibiscus rosa�sinensis leaf extract as coagulant aid in leachate treat�ment" Applied Water Science 2: 293–298. DOI: 10.1007/s13201-012-0049-y.
- [32] Z. Zhang, S. Xia, J. Zhao, and J. Zhang, (2010) “Char�acterization and flocculation mechanism of high-efficiency microbial flocculant TJ-F1 from Proteus mirabilis" Col�loids and Surfaces B: Biointerfaces 75(1): 247–251. DOI: 10.1016/j.colsurfb.2009.08.038.
- [33] A. F. Santos, L. A. Luz, A. C. Argolo, J. A. Teixeira, P. M. Paiva, and L. C. Coelho, (2009) “Isolation of a seed coagulant Moringa oleifera lectin" Process bio�chemistry 44(4): 504–508. DOI: 10.1016/j.procbio.2009.01.002.