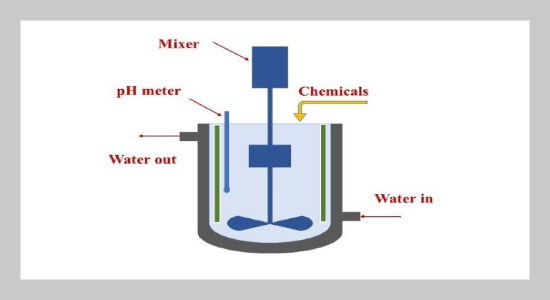
REFERENCES
- [1] D. Grossmann, H. Köser, R. Kretschmer, and M. Porobin, (2001) “Treatment of diglyme containing wastewater by advanced oxidation - Process design and optimisation" Water Science and Technology 44(5): 287–293. DOI: 10.2166/wst.2001.0308.
- [2] M. Singh and R. Srivastava, (2011) “Sequencing batch reactor technology for biological wastewater treatment: A review" Asia-Pacific Journal of Chemical Engineering 6(1): 3–13. DOI: 10.1002/apj.490.
- [3] T. Stephenson, K. Brindle, S. Judd, and B. Jefferson. Membrane bioreactors for wastewater treatment. IWA publishing, 2000.
- [4] W. Somasiri, X.-F. Li, W.-Q. Ruan, and C. Jian, (2008) “Evaluation of the efficacy of upflow anaerobic sludge blanket reactor in removal of colour and reduction of COD in real textile wastewater" Bioresource Technology 99(9): 3692–3699. DOI: 10.1016/j.biortech.2007.07.024.
- [5] M. Latif, R. Ghufran, Z. Wahid, and A. Ahmad, (2011) “Integrated application of upflow anaerobic sludge blanket reactor for the treatment of wastewaters" Water Research 45(16): 4683–4699. DOI: 10.1016/j.watres.2011. 05.049.
- [6] W.-t. Zhao, X. Huang, and D.-j. Lee, (2009) “Enhanced treatment of coke plant wastewater using an anaerobic- anoxic-oxic membrane bioreactor system" Separation and Purification Technology 66(2): 279–286. DOI: 10. 1016/j.seppur.2008.12.028.
- [7] V. Leite, S. Prasad, W. Lopes, J. de Sousa, and A. Barros, (2013) “Study on ammonia stripping process of leachate from the packed towers" Journal of Urban and Environmental Engineering 7(2): 215–222. DOI: 10.4090/juee.2013.v7n2.215222.
- [8] S. Guštin and R. Marinšek-Logar, (2011) “Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent" Process Safety and Environmental Protection 89(1): 61–66. DOI: 10.1016/j.psep.2010.11.001.
- [9] X. Li and Q. Zhao, (2003) “Recovery of ammonium- nitrogen from landfill leachate as a multi-nutrient fertilizer" Ecological Engineering 20(2): 171–181. DOI: 10.1016/S0925-8574(03)00012-0.
- [10] Q. Wu and P. Bishop, (2004) “Enhancing struvite crystallization from anaerobic supernatant" Journal of Environmental Engineering and Science 3(1): 21–29. DOI: 10.1139/S03-050.
- [11] S. Wongkiew, Z. Hu, J. W. Lee, K. Chandran, H. T. Nhan, K. R. Marcelino, and S. K. Khanal, (2021) “Nitrogen Recovery via Aquaponics–Bioponics: Engineering Considerations and Perspectives" ACS ES&T Engineering 1(3): 326–339.
- [12] R. Yu, J. Geng, H. Ren, Y. Wang, and K. Xu, (2013) “Struvite pyrolysate recycling combined with dry pyrolysis for ammonium removal from wastewater" Bioresource Technology 132: 154–159. DOI: 10.1016/j.biortech. 2013.01.015.
- [13] N. Acelas, E. Flórez, and D. López, (2015) “Phosphorus recovery through struvite precipitation from wastewater: effect of the competitive ions" Desalination and Water Treatment 54(9): 2468–2479. DOI: 10.1080/19443994. 2014.902337.
- [14] A. Korchef, H. Saidou, and M. Amor, (2011) “Phosphate recovery through struvite precipitation by CO2 removal: Effect of magnesium, phosphate and ammonium concentrations" Journal of Hazardous Materials 186(1): 602–613. DOI: 10.1016/j.jhazmat.2010.11.045.
- [15] B. Tansel, G. Lunn, and O. Monje, (2018) “Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions" Chemosphere 194: 504–514. DOI: 10.1016/j.chemosphere.2017.12.004.
- [16] M. E. Trenkel. Slow-and controlled-release and stabilized fertilizers: an option for enhancing nutrient use efficiency in agriculture. IFA, International fertilizer industry association, 2010.
- [17] E. Tarragó, S. Puig, M. Ruscalleda, M. Balaguer, and J. Colprim, (2016) “Controlling struvite particles’ size using the up-flow velocity" Chemical Engineering Journal 302: 819–827. DOI: 10.1016/j.cej.2016.06.036.
- [18] F. Ramírez, V. González, M. Crespo, D. Meier, O. Faix, and V. Zúñiga, (1997) “Ammoxidized kraft lignin as a slow-release fertilizer tested on Sorghum vulgare" Bioresource Technology 61(1): 43–46. DOI: 10.1016/S0960 8524(97)84697-4.
- [19] B. Azeem, K. Kushaari, Z. Man, A. Basit, and T. Thanh, (2014) “Review on materials methods to produce controlled release coated urea fertilizer" Journal of Controlled Release 181(1): 11–21. DOI: 10.1016/j. jconrel.2014.02.020.
- [20] R. L. Mason, R. F. Gunst, and J. L. Hess. Statistical design and analysis of experiments: with applications to engineering and science. 474. John Wiley & Sons, 2003.
- [21] S. Zhou and Y. Wu, (2012) “Improving the prediction of ammonium nitrogen removal through struvite precipitation" Environmental Science and Pollution Research 19(2): 347–360. DOI: 10.1007/s11356-011-0520- 6.
- [22] Z.-L. Ye, S.-H. Chen, S.-M. Wang, L.-F. Lin, Y.-J. Yan, Z.-J. Zhang, and J.-S. Chen, (2010) “Phosphorus recovery from synthetic swine wastewater by chemical precipitation using response surface methodology" Journal of Hazardous Materials 176(1-3): 1083–1088.
- [23] G. Jia, H. Zhang, J. Krampe, T. Muster, B. Gao, N. Zhu, and B. Jin, (2017) “Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent" Journal of Cleaner Production 147: 297–305. DOI: 10.1016/j. jclepro.2017.01.116.
- [24] S. Kumari, S. Jose, and S. Jagadevan, (2019) “Optimization of phosphate recovery as struvite from synthetic distillery wastewater using a chemical equilibrium model" Environmental Science and Pollution Research 26(29): 30452–30462. DOI: 10.1007/s11356-019- 06152-4.
- [25] S. Polat and P. Sayan, (2019) “Application of response surface methodology with a Box–Behnken design for struvite precipitation" Advanced Powder Technology 30(10): 2396–2407. DOI: 10.1016/j.apt.2019.07.022.
- [26] M. Rahman, M. Salleh, and T. D. U. Rashid, (2018) “Recovery of nitrogen and phosphorus from synthetic wastewater through crystallization process" Journal of Desalination and Water Purification 3: 11–16.
- [27] S. Lee, S. Weon, C. Lee, and B. Koopman, (2003) “Removal of nitrogen and phosphate from wastewater by addition of bittern" Chemosphere 51(4): 265–271. DOI: 10.1016/S0045-6535(02)00807-X.
- [28] X. Hao, C. Wang, M. C. Van Loosdrecht, and Y. Hu. Looking beyond struvite for P-recovery. 2013.
- [29] A. Adnan, F. A. Koch, and D. S. Mavinic, (2003) “Pilot-scale study of phosphorus recovery through struvite crystallization–II: Applying in-reactor supersaturation ratio as a process control parameter" Journal of Environmental Engineering and Science 2(6): 473–483.
- [30] E. Munch and K. Barr, (2001) “Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams" Water Research 35(1): 151–159.
- [31] S. Tang, D. Yuan, Y. Rao, J. Zhang, Y. Qu, and J. Gu, (2018) “Evaluation of antibiotic oxytetracycline removal in water using a gas phase dielectric barrier discharge plasma" Journal of Environmental Management 226: 22–29. DOI: 10.1016/j.jenvman.2018.08.022.
- [32] W. E. Federation, A. Association, et al., (1998) “Standard methods for the examination of water and wastewater" American Public Health Association (APHA): Washington, DC, USA:
- [33] W. Gong, Y. Li, L. Luo, X. Luo, X. Cheng, and H. Liang, (2018) “Application of struvite-MAP crystallization reactor for treating cattle manure anaerobic digested slurry: Nitrogen and phosphorus recovery and crystal fertilizer efficiency in plant trials" International Journal of Environmental Research and Public Health 15(7): DOI: 10.3390/ijerph15071397.
- [34] A. Ahmad and A. Idris, (2014) “Release and recovery of phosphorus from wastewater treatment sludge via struvite precipitation" Desalination and Water Treatment 52(28-30): 5696–5703. DOI: 10.1080/19443994.2013. 813101.
- [35] S. Shaddel, S. Ucar, J.-P. Andreassen, and S. Sterhus, (2019) “Engineering of struvite crystals by regulating supersaturation - Correlation with phosphorus recovery, crystal morphology and process efficiency" Journal of Environmental Chemical Engineering 7(1): DOI: 10. 1016/j.jece.2019.102918.
- [36] S. Daneshgar, A. Buttafava, D. Capsoni, A. Callegari, and A. Capodaglio, (2018) “Impact of pH and ionic molar ratios on phosphorous forms precipitation and recovery from different wastewater sludges" Resources 7(4): DOI: 10.3390/resources7040071.
- [37] B. Etter, E. Tilley, R. Khadka, and K. Udert, (2011) “Low-cost struvite production using source-separated urine in Nepal" Water Research 45(2): 852–862. DOI: 10 . 1016/j.watres.2010.10.007.
- [38] W. M. Haynes, (2014) “CRC Handbook of chemistry and physics, CRC Press" Inc, Boca Raton, FL:
- [39] B. Li, H. Huang, I. Boiarkina, W. Yu, Y. Huang, G. Wang, and B. Young, (2019) “Phosphorus recovery through struvite crystallisation: Recent developments in the understanding of operational factors" Journal of Environmental Management 248: DOI: 10.1016/j. jenvman.2019.07.025.