Jyisy Yang This email address is being protected from spambots. You need JavaScript enabled to view it.1 and Wan-Chan Chen2 1Department of Chemistry National Chung-Hsing University Taichung, Taiwan 402, R.O.C.
2Department of Chemistry Chung-Yuan Christian University Chungli, Taiwan 320, R.O.C.
Received:
October 16, 2002
Accepted:
November 5, 2002
Publication Date:
December 1, 2002
Download Citation:
||https://doi.org/10.6180/jase.2002.5.4.05
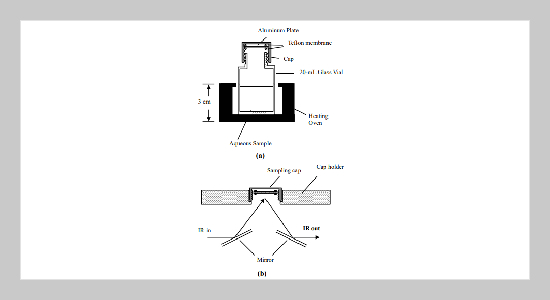
In this paper, a new and simple IR sensing device was proposed for the determination of semi-volatile chlorinated aromatic compounds in aqueous solutions. In this sensing device, a 20-mL glass vial was modified and used to analyze aqueous solutions by conventional Fourier-Transform Infrared (FT-IR) spectrometer. An aluminum plate coated with hydrophobic film was placed on the top of the cap of the sample vial for absorption of analytes evaporated from aqueous solutions. After end of absorption process, this cap was moved to the FT-IR spectrometer for detection of analytes in reflection-absorption (RA) mode. To accelerate the speed in evaporation of analytes from aqueous solutions, the samples were heated to various degree of temperature. Meanwhile, factors, such as speed of stirring, volume of headspace, film thickness of hydrophobic film, volatilities of analytes, and concentrations of analytes, were also examined in order to optimize the analytical conditions. The results indicated that speed to reach the equilibrium condition were highly fast and could be done within 10 minutes of extraction time. After examination of compounds with different vapor pressures, the analytical results indicated that this method was applicable to examine compounds with vapor pressure lower than 1 Torr. Using the optimal conditions found in this work, the detection limits for semi-volatile aromatic compounds were around 200 ppb and the regression coefficients of the standard curves for examined compounds were larger than 0.992 in the concentration range of 5 ppm to 100 ppm.ABSTRACT
Keywords:
Fourier-Transform Infrared (FT-IR), Reflection-Absorption, Semi-Volatile Compounds, Chlorinated Aromatic Compounds
REFERENCES