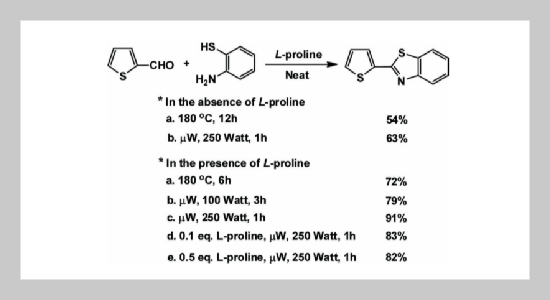
REFERENCES
- [1] Bradshaw, T. D., Wrigley, S., Shi, D. F., Schulz, R. J., Paull, K. D. and Stevens, M. F. G., “2-(4-Aminophenyl)Benzothiazoles: Novel Agents with Selective Profiles of in Vitro Anti-Tumour Activity,” Br. J. Cancer, Vol. 77, pp. 745�752 (1998).
- [2] Beneteau, V., Besson, T., Guillard, J., Leonce, S. and Pfeiffer, B., “Synthesis and in Vitro Antitumour Evaluation of Benzothiazole-2-Carbonitrile Derivatives,” Eur. J. Med. Chem., Vol. 34, pp. 1053�1060 (1999).
- [3] Malcolm, F. G. and Westwell, A. D., “Antitumour Benzothiazoles. Part 20: 3’-Cyano and 3’-AlkynylSubstituted 2-(4’-Aminophenyl)Benzothiazoles as New Potent and Selective Analogues,” Bioorg. Med. Chem. Lett., Vol. 13, pp. 471�474 (2003).
- [4] Costa, S. P. G., Ferreira, J. A., Kirsch, G. and Oliveira-Campos, M. F. J., “New Fluorescent 1,3- Benzothiazoles by the Reaction of Heterocyclic Aldehydes with o-Aminobenzenethiol,” Chem. Res., pp. 314�315 (1997).
- [5] Heynderickx, A., Guglielmetti, R., Dubest, R., Aubard, J. and Samat, A., “Sulfinyl- and Sulfonyl-Substituted 2-Benzylbenzoxazoles and 2-Benzylbenzothiazoles as Potential Photochromic Compounds,” Synthesis, pp. 1112�1116 (2003).
- [6] Batista, R. M. F., Costa, S. P. G. and Raposo, M. M. M., “Synthesis of New Fluorescent 2-(2�,2�-Bithienyl)- 1,3-Benzothiazoles,” Tetrahedron Lett., Vol. 45, pp. 2825�2828 (2004).
- [7] Kodomari, M., Tamura, Y. and Aoyama, T., “SolventFree Synthesis of 2-Aryl and 2-Alkylbenzothiazoles on Silica Gel under Microwave Irradiation,” Synth. Commun., Vol. 34, pp. 3029�3036 (2004).
- [8] Itoh, T., Nagata, K., Ishikawa, H. and Ohsawa, A., “Synthesis of 2-Arylbenzothiazoles from 2-Aminobenzenethiol and Aryl Aldehydes Catalyzed by Scandium Triflate,” Heterocycles, Vol. 62, pp. 197�201 (2004).
- [9] Ranu, B. C., Janna, R. and Dey, S., “An Efficient and Green Synthesis of 2-Arylbenzothiazoles in an Ionic Liquid, [pmIm]Br under Microwave Irradiation,” Chem. Lett., Vol. 33, pp. 274�275 (2004).
- [10] Li. Y., Wang, Y.-L. and Wang, J.-Y., “A Simple Iodine-Promoted Synthesis of 2-Substituted Benzothiazoles by Condensation of Aldehydes with 2-Aminothiophenol,” Chem. Lett., Vol. 35, pp. 460�461 (2006).
- [11] Brembilla, A., Roizard, D. and Lochon, P., “1-Methyl-2-Pyrrolidinone: A Well-Adapted Solvent in the Benzothiazoles Synthesis,” Synth. Commun., Vol. 20, pp. 3379�3384 (1990).
- [12] Laskar, I. R. and Chen, T. M., “Tuning of Wavelengths: Synthesis and Photophysical Studies of Iridium Complexes and Their Applications in Organic Light Emitting Devices,” Chem. Mater., Vol. 16, pp. 111�117 (2004).
- [13] Mourtas, S., Gatos, D. and Barlos, K., “Solid Phase Synthesis of Benzothiazolyl Compounds,” Tetrahedron Lett., Vol. 42, pp. 2201�2204 (2001).
- [14] Chen, C. and Chen, Y. J., “Liquid-Phase Synthesis of 2-Substituted Benzimidazoles, Benzoxazoles and Benzothiazoles,” Tetrahedron Lett., Vol. 45, pp. 113�115 2004.
- [15] Chakraborti, A. K., Selvam, C., Kaur, G. and Bhagat, S., “An Efficient Synthesis of Benzothiazoles by Direct Condensation of Carboxylic Acids with 2-Aminothiophenol under Microwave Irradiation,” Synlett, pp. 851�855 (2004).
- [16] Yildiz-Oren, I., Yalcin, I., Aki-Sener, E. and Ucarturk, N., “Synthesis and Structure � Activity Relationships of New Antimicrobial Active Multisubstituted Benzazole Derivatives,” Eur. J. Med. Chem., Vol. 39, pp. 291�298 (2004).
- [17] Chakraborti, A. K., Rudrawar, S., Kaur, G. and Bharma, L., “An Efficient Conversion of Phenolic Esters to Benzothiazoles under Mild and Virtually Neutral Conditions,” Synlett, pp. 1533�1536 (2004).
- [18] Matsushita, H., Lee, S. H., Joung, M., Clapkam, B. and Janda, K. D., “Smart Cleavage Reactions: The Synthesis of Benzimidazoles and Benzothiazoles from Polymer-Bound Esters,” Tetrahedron Lett., Vol. 45, pp. 313�316 (2004).
- [19] Ben-Alloum, A., Bakkas, S. and Soufiaoui, M., “Nouvelle Voie de Synthèse des 2-Arylbenzothiazoles Transfert D'electrons Activé par Micro-Ondes,” Tetrahedron Lett., Vol. 38, pp. 6395�6396 (1997).
- [20] Abbotto, A., Bradamante, S., Facchetti, A. and Pagani, G. A., “Metal Chelation Aptitudes of Bis(o-Azaheteroaryl)Methanes as Tuned by Heterocycle Charge Demands,” J. Org. Chem., Vol. 67, pp. 5753�5772 (2002).
- [21] Tale, R. H., “Novel Synthesis of 2-Arylbenzothiazoles Mediated by Ceric Ammonium Nitrate (CAN),” Org. Lett., Vol. 4, pp. 1641�1642 (2002).
- [22] Shi, D. F., Bradshaw, T. D., Wrigley, S., McCall, C. J., Lelieveld, P., Fichtner, I. and Stevens, M. F. G., “Antitumor Benzothiazoles. 3. Synthesis of 2-(4-Aminophenyl) Benzothiazoles and Evaluation of Their Activities Against Breast Cancer Cell lines in Vitro Andin Vivo,” J. Med. Chem., Vol. 39, pp. 3375�3384 (1996).
- [23] Hutchinson, I., Stevens, M. F. G. and Westwell, A. D., “The Regiospecific Synthesis of 5- and 7-Monosubstituted and 5,6-Disubstituted 2-Arylbenzothiazoles,” Tetrahedron Lett., Vol. 41, pp. 425�428 (2000).
- [24] Benedi, C., Bravo, F., Uriz, P., Fernandez, E., Claver, C. and Castillon, S., “Synthesis of 2-Substituted-Benzothiazoles by Palladium-Catalyzed Intramolecular Cyclization of o-Bromophenylthioureas and o-Bromophenylthioamides,” Tetrahedron Lett., Vol. 44, pp. 6073�6077 (2003).
- [25] Joyce, L. L., Evindar, G. and Batey, R. A., “Copperand Palladium-Catalyzed Intramolecular C�S Bond Formation: A Convenient Synthesis of 2-Aminobenzothiazoles,” Chem. Commun., pp. 446�447 (2004).
- [26] Mu, X. J., Zou, J. P., Zeng, R. S. and Wu, J. C., “Mn(III)-Promoted Cyclization of Substituted Thioformanilides under Microwave Irradiation: A New Reagent for 2-Substituted Benzothiazoles,” Tetrahedron Lett., Vol. 46, pp. 4345�4347 (2005).
- [27] Moghaddam, F. M. and Boeini, H. Z., “Oxidative Cyclization of Thiobenzanilides to Benzothiazoles Using N-Benzyl-DABCO Tribromide under Mild Conditions,” Synlett, pp. 1612�1614 (2005).
- [28] Zificsak, C. A. and Hlasta, D. J., “Current Methods for the Synthesis of 2-Substituted Azoles,” Tetrahedron, Vol. 60, pp. 8991�9016 (2004).
- [29] Majo, V. J., Prabhakaran, J., Mann, J. J. and Kumar, J. S. D., “An Efficient Palladium Catalyzed Synthesis of 2-Arylbenzothiazoles,” Tetrahedron Lett., Vol. 44, pp. 8535�8537 (2003).
- [30] Belfield, K. D., Schafer, K. J., Mourad, W. and Reinhardt, B. A., “Synthesis of New Two-Photon Absorbing Fluorene Derivatives via Cu-Mediated Ullmann Condensations,” J. Org. Chem., Vol. 65, pp. 4475�4481 (2000).
- [31] Alagille, D., Baldwin, R. M. and Tamagnan, G. D., “One-Step Synthesis of 2-Arylbenzothiazole (BTA) and 2-Arylbenzoxazole Precursors for in Vivo Imaging of -Amyloid Plaques,” Tetrahedron Lett., Vol. 46, pp. 1349�1351 (2005).
- [32] Lee, A. S.-Y., Tsao, K.-W., Chang, Y.-T. and Chu, S.-F., “L-Proline Catalyzed Intramolecular Cyclization of 5-Hydroxypentene to -Halogenated Tetrahydrofuran,” Tetrahedron Lett., Vol. 48, pp. 6790�6793 (2007).