Chih-Sheng Yu This email address is being protected from spambots. You need JavaScript enabled to view it.1, Ming-Yu Lin1, Heng-Tsang Hu1, Yi-Chiuen Hu1 and Haiao-Yu Chou1 1Instrument Technology Research Center, National Applied Research Laboratories Hsinchu, Taiwan 300, R.O.C.
Received:
January 15, 2005
Accepted:
June 2, 2005
Publication Date:
September 1, 2005
Download Citation:
||https://doi.org/10.6180/jase.2005.8.3.06
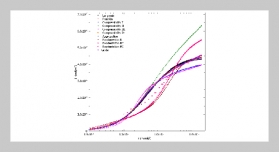
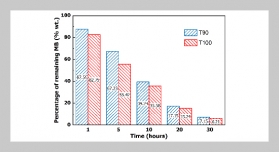
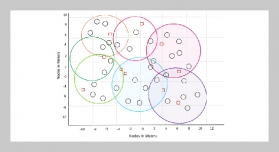
We characterize a droplet-based biochemical reaction device with the features of droplet self-positioning, self-mixing and self-alignment optics on a micro-patterning PDMS chip. The phenomenon of droplet self-positioning is explained as follows: the different texture zones generate gradient of surface tension force to manipulate droplet. Droplet-based reagents can be transported, precisely positioning, and mixed on the detection zone without any power source. The study was carried out to experimentally determined triglycerides (TG) using this droplet manipulation device with correlation coefficient 0.922 from 19 mg/dl to 480 mg/dl.ABSTRACT
Keywords:
Droplet Manipulation, Droplet Self-mixing, Triglycerides Assay
REFERENCES