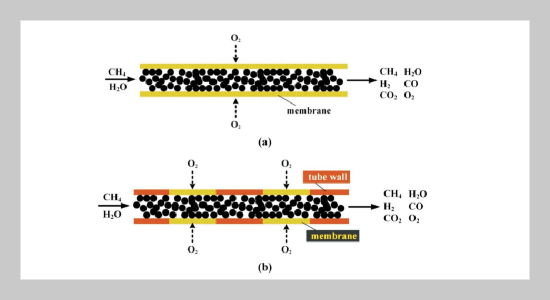
REFERENCES
- [1] Ulber, D., “A Guide to Methane Reforming,” Chemical Engineering, January, pp. 40�46 (2015).
- [2] Rice,S. F. and Mann, D. P., Autothermal Reforming of Natural Gas to Synthesis Gas,SAND2007-2331, Sandia National Laboratories, U.S. Department of Commerce(2007).
- [3] Marcano, J. G. S. and Tsotsis, T. T., Catalytic Membranes and Membrane Reactors, Wiley-VCH Verlag GmbH, WeinheimGermany(2002).
- [4] Coronas,J.andSantamaría,J.,“CatalyticReactorsBased on Porous Ceramic Membranes,” Catalysis Today, Vol. 51, pp. 377�389 (1999). doi: 10.1016/S0920-5861 (99)00090-5
- [5] Rodríguez, M. L., Ardissone, D. E., Opez, E. L., Pedernera, M. N. and Borio, D. O., “Reactor Designs for Ethylene Production via Ethane Oxidative Dehydrogenation: Comparison of Performance,” Industrial and Engineering Chemistry Research, Vol. 50, pp. 2690�2697 (2011). doi: 10.1021/ie100738q
- [6] Rodríguez, M. L., Pedernera, M. N. and Borio, D. O., “Two Dimensional Modeling of a Membrane Reactor for ATR of Methane,” Catalysis Today, Vol. 193, pp. 137�144 (2012). doi: 10.1016/j.cattod.2012.04.010
- [7] Coronas,J.,Menéndez,M.andSantamaría,J.,“Use of a Ceramic Membrane Reactor for the Oxidative Dehydrogenation of Ethane to Ethylene and Higher Hydrocarbons,” Industrial and Engineering Chemistry Research, Vol. 34, pp. 4229�4234 (1995). doi: 10.1021/ ie00039a011
- [8] Ávila-Neto, C. N., Dantas, S. C., Silva, F. A., Franco, T. V., Romanielo, L. L., Hori, C. E. and Assis, A. J., “Hydrogen Production fromMethane Reforming: ThermodynamicAssessmentandAutothermalReactorDesign,” Journal of Natural Gas Science and Engineering, Vol. 1, pp. 205�215 (2009). doi: 10.1016/j.jngse. 2009.12.003
- [9] Sinaei Nobandegani, M., Sardashti Birjandi, M. R., Darbandi, T., Khalilipour, M. M., Shahraki, F. and Mohebbi-Kalhori, D., “An Industrial Steam Methane Reformer Optimization Using Response Surface Methodology,” Journal of Natural Gas Science and Engineering, Vol. 36, pp. 540�549 (2016). doi: 10.1016/j. jngse.2016.10.031
- [10] Shahhosseini, H.R.,Farsi,M.and Eini,S.,“Multi-objective Optimization of Industrial Membrane SMR to Produce Syngas for Fischer-Tropsch Production Using NSGA-II and Decision Makings,” Journal of Natural Gas Science and Engineering, Vol. 32, pp. 222� 238 (2016). doi: 10.1016/j.jngse.2016.04.005
- [11] Deb, K., Patap, A., Agarwal, S. and Meyarivan, T., “A Fast and Elitist Multiobjective Genetic Algorithm: NSGA-II,” IEEE Transactions on Evolutionary Computation, Vol. 6, pp. 182�197 (2002). doi: 10.1109/ 4235.996017
- [12] De Groote, A. M. and Froment, G. F., “Simulation of the Catalytic Partial Oxidation of Methane to Synthesis Gas,” Applied Catalysis A: General, Vol. 138, pp. 245�264 (1996).doi:10.1016/0926-860X(95)00299-5
- [13] Ergun, S., “Fluid Flow through Packed Columns,” Chem. Eng. Prog., Vol. 48, pp. 89�94 (1952).
- [14] Xu,J.andFroment,G.F.,“MethaneSteamReforming, MethanationandWater-gasShift:I.IntrinsicKinetics,” AIChE Journal, Vol. 35, pp. 88�96 (1989). doi: 10. 1002/aic.690350109
- [15] Mallada, R., Pedernera, M., Menéndez, M. and Santamaria, J., “Synthesis of Maleic Anhydride in an InertMembraneReactor.EffectofReactorConfiguration,” Industrial and Engineering Chemistry Research, Vol. 39, pp. 620�625 (2000). doi: 10.1021/ie9905310
- [16] Pedernera, M., Mallada, R., Menéndez, M. and Santamaria, J., “Simulation of an Inert Membrane Reactor for the Synthesis of Maleic Anhydride,” AIChE Journal,Vol.46,pp.2489�2498(2000). doi:10.1002/ aic.690461215
- [17] Rodríguez, M. L., Ardissone, D. E., Lemonidou, A. A., Heracleous, E., López, E., Pedernera, M. N. and Borio, D. O., “Simulation of a Membrane Reactor for the Catalytic Oxidehydrogenation of Ethane,” Industrial and Engineering Chemistry Research, Vol. 48, pp. 1090�1095 (2009). doi: 10.1021/ie800564v
- [18] Froment, G. F. and Bischoff, K. B., Chemical Reactor Analysis and Design, Wiley, New York, USA(1990).