Chang-Ching You1 , Chin-Bin Lin1 , Yang-Min Liang2 , Chi-Wang Li This email address is being protected from spambots. You need JavaScript enabled to view it.2 and Hui-Chung Hsueh1 1Department of Mechanical and Electro-Mechanical Engineering, Tamkang University, Tamsui, Taiwan 251, R.O.C.
2Department of Water Resources and Environmental Engineering, Tamkang University, Tamsui, Taiwan 251, R.O.C.
Received:
November 6, 2014
Accepted:
February 3, 2015
Publication Date:
March 1, 2015
Download Citation:
||https://doi.org/10.6180/jase.2015.18.1.02
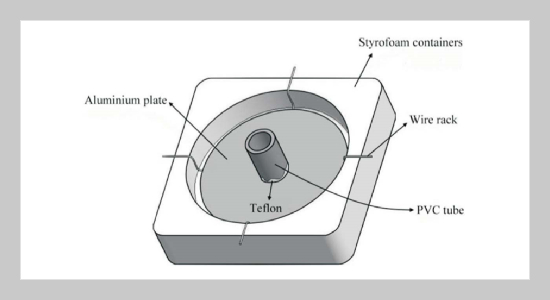
Silver chloride thin film (SCTF) with high specific surface area was synthesized through precipitation reaction by adding sodium chloride solution on top of frozen silver nitrate solution. Effects of precipitation time and silver nitrate concentration on the morphology of SCTFs were investigated. SEM images show that small crystal AgCl grains forming rod-like structure appeared on the bottom surface of the SCTF. After exposure of SCTFs to UV light, clusters of silver atoms were formed on the surface of SCTF as indicated by XRD analysis. Six SCTFs were attached to interior wall of the photo-reactor using double sided adhesive tape for investigation of photocatalytic property of the films. Almost completed decolorization of orange II dye by SCTF can be achieved under UV light illumination within two hours. SCTFs made under different initial silver nitrate solution concentrations of 8.4 M, 4.2 M and 3.6 M have little impact on dye decolorization efficiency. The photocatalytic degradation of orange II dye by SCTF under visible light illumination is less efficient than that under UV light. Around 31% of color can be removed after two hours.ABSTRACT
Keywords:
Precipitation, Silver Chloride Thin Film, Photocatalytic, Dye, Photosensitivity
REFERENCES