REFERENCES
- [1] D. Pradhan, K. A. Suri, D. K. Pradhan, and P. Biswasroy, (2013) “Golden Heart of the Nature : Piper betle L" Journal of Pharmacognosy and Phytochemistry 1(6): 147–167.
- [2] D. Alighiri, E. Cahyono,W. Tirza Eden, E. Kusuma, and K. Imam Supardi, (2018) “Study on the improvement of essential oil quality and its repellent activity of betel leaves oil (Piper betle l.) from Indonesia" Orient. J. Chem. 34(6): 2913–2926.
- [3] D. Gupta and A. Singh, (2016) “Piper betle and some Indian Plant for Antidepressant Activity: A Review" Research Journal of Pharmaceutical, Biological and Chemical Sciences (7): 1670–1678.
- [4] N. A. Ghazali, A. Elmy, L. C. Yuen, N. Z. Sani, S. Das, F. Suhaimi, R. Yusof, N. H. Yusoff, and Z. C. Thent, (2016) “Piper betel leaves induces wound healing activity via proliferation of fibroblasts and reducing 11β hydroxysteriod dehydrogenase-1 expression in diabetic rat" J. Ayurveda Integr. Med. 7(4): 198–208. DOI: 10.1016/j.jaim.2016.08.008.
- [5] E. M. Jamelarin and L. O. Balinado, (2019) “Evaluation of antibacterial activity of crude aqueous, ethanolic and methanolic leaf extracts of Piper retrofractum Vahl. And Piper betle L" Asian Journal of Biological and Life Sciences 8(2): 63–67.
- [6] N. I. Azahar, N. M. Mokhtar, and M. A. Arifin, (2020) “Piper betle: a review on its bioactive compounds, pharmacological properties, and extraction process" IOP Conf. Ser. Mater. Sci. Eng. 991(1): 012044.
- [7] M. Madhumita, P. Guha, and A. Nag, (2019) “Extraction of betel leaves (Piper betle L.) essential oil and its bio-actives identification: Process optimization, GC-MS analysis and anti-microbial activity" Ind. Crops Prod. 138(111578): 111578. DOI: 10.1016/j.indcrop.2019.111578.
- [8] U. Taukoorah, N. Lall, and F. Mahomoodally, (2016) “Piper betle L. (betel quid) shows bacteriostatic, additive, and synergistic antimicrobial action when combined with conventional antibiotics" S. Afr. J. Bot. 105: 133–140. DOI: 10.1016/j.sajb.2016.01.006.
- [9] S. Basak and P. Guha, (2016) “Betel leaf ( Piper betle L.) Essential Oil Microemulsion: Characterization and Antifungal Activity on Growth, and Apparent Lag Time of Aspergillus flavus in Tomato Paste" Science and Technology 75: 616–623. DOI: 10.1016/j.lwt.2016.10.021.
- [10] N. Balasundram, K. Sundram, and S. Samman, (2006) “Phenolic compounds in plants and agri-industrial byproducts: Antioxidant activity, occurrence, and potential uses" Food Chem. 99(1): 191–203. DOI: 10.1016/j.foodchem.2005.07.042.
- [11] A. Ali, X. Y. Lim, and P. F. Wahida, (2018) “The fundamental study of antimicrobial activity of Piper betle extract in commercial toothpastes" J. Herb. Med. 14:29–34. DOI: 10.1016/j.hermed.2018.08.001.
- [12] L. Nouri, A. Mohammadi Nafchi, and A. A. Karim, (2014) “Phytochemical, antioxidant, antibacterial, and α-amylase inhibitory properties of different extracts from betel leaves" Ind. Crops Prod. 62: 47–52. DOI: 10.1016/j.indcrop.2014.08.015.
- [13] M. Anugrahwati, T. Purwaningsih, Rustina, J. A. Manggalarini, N. B. Alnavis, D. N. Wulandari, and H. D. Pranowo, (2016) “Extraction of ethanolic extract of red betel leaves and its cytotoxicity test on HeLa cells" Procedia Eng. 148: 1402–1407. DOI: 10.1016/j.proeng.2016.06.569.
- [14] L. Muruganandami, A. Krishna, and J. Reddy, (2017) “Optimization Studies on Extraction of Phytocomponents from Betel Leaves" Resource-Efficient Technologies 3(4): 385–393.
- [15] Y. Li, G. K. Skouroumounis, G. M. Elsey, and D. K. Taylor, (2011) “Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols" Food Chemistry 129(2): 570–576. DOI: 10.1016/j.foodchem.2011.04.068.
- [16] A. A. Jovanovic, V. B. Ðord¯evic, G. M. Zdunic, D. S. Pljevljakusic, K. P. Savikin, D. M. God¯evac, and B. M. Bugarski, (2017) “Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques" Separation and Purification Technology 179: 369–380. DOI: 10.1016/j.seppur.2017.01.055.
- [17] M. Herrero, J. A. Mendiola, A. Cifuentes, and E. Ibáñez, (2010) “Supercritical fluid extraction: Recent advances and applications" Journal of Chromatography a 1217(16): 2495–2511. DOI: 10.1016/j.chroma.2009.12.019.
- [18] S. O. Essien, B. Young, and S. Baroutian, (2020) “Recent advances in subcritical water and supercritical carbon dioxide extraction of bioactive compounds from plant materials" Trends Food Sci. Technol. 97: 156–169. DOI: 10.1016/j.tifs.2020.01.014.
- [19] F. Chemat and J. Strube. Green extraction of natural products: theory and practice. John Wiley & Sons, 2015. DOI: 10.1002/9783527676828.
- [20] J. Zhang, C. Wen, H. Zhang, Y. Duan, and H. Ma, (2020) “Recent advances in the extraction of bioactive compounds with subcritical water: A review" Trends Food Sci. Technol. 95: 183–195. DOI: 10.1016/j.tifs.2019.11.018.
- [21] N. Nastic, J. Svarc-Gajic, C. Delerue-Matos, M. F. Barroso, C. Soares, M. M. Moreira, S. Morais, P. Maskovic, V. G. Srcek, I. Slivac, K. Radosevic, and M. Radojkovic, (2018) “Subcritical water extraction as an environmentally-friendly technique to recover bioactive compounds from traditional Serbian medicinal plants" Industrial Crops and Products 111: 579–589. DOI: 10.1016/j.indcrop.2017.11.015.
- [22] S. Essien, B. Young, and S. Baroutian, (2020) “Subcritical water extraction for selective recovery of phenolic bioactives from k¯anuka leaves" The Journal of Supercritical Fluids 158: 104721. DOI: 10.1016/j.supflu.2019.104721.
- [23] A. M. Silva, A. S. Luís, M. M. Moreira, R. Ferraz, T. Brezo-Borjan, J. Švarc-Gaji´c, P. C. Costa, C. Delerue-Matos, and F. Rodrigues, (2022) “Influence of temperature on the subcritical water extraction of Actinidia arguta leaves: A screening of pro-healthy compounds" Sustainable Chemistry and Pharmacy 25: 100593. DOI: 10.1016/j.scp.2021.100593.
- [24] D. Šeremet, S. Joki´c, K. Aladi´c, A. V. Cebin, N. Božac, A. Mandura, and D. Komes, (2021) “Optimization of heat-, microwave-assisted and subcritical water extraction of phenolic compounds from ground ivy (Glechoma hederacea L.) using response surface methodology" Journal of Applied Research on Medicinal and Aromatic Plants 25: 100346.
- [25] Y. Wu, Y. Yu, H. Wang, Y. Jiang, Z. Yang, J. Zhou, and L. Zhang, (2021) “Preparation of paeoniflorin from the stems and leaves of Paeonia lactiflora Pall.‘Zhongjiang’through green efficient microwave assisted extraction and subcritical water extraction" Industrial Crops and Products 163: 113332. DOI: 10.1016/j.indcrop.2021.113332.
- [26] S. M. Zakaria, S. M. M. Kamal, M. R. Harun, R. Omar, and S. I. Siajam, (2017) “Subcritical water technology for extraction of phenolic compounds from Chlorella sp. Microalgae and assessment on its antioxidant activity" Molecules 22(7): 1105. DOI: 10.3390/molecules22071105.
- [27] E. Mlyuka, S. Zhang, L.Wang, Z. Zheng, and J. Chen, (2016) “Characteristics of subcritical water extraction and kinetics of pentacyclic triterpenoids from dry loquat (Eriobotrya japonica) leaves" International Journal of Food Engineering 12(6): 547–555.
- [28] Z. Ju and L. R. Howard, (2005) “Subcritical water and sulfured water extraction of anthocyanins and other phenolics from dried red grape skin" Journal of Food Science 70(4): S270–S276. DOI: 10.1111/j .1365- 2621.2005.tb07202.x.
- [29] L. Ramos, E. M. Kristenson, and U. T. Brinkman, (2002) “Current use of pressurised liquid extraction and subcritical water extraction in environmental analysis" Journal of Chromatography A 975(1): 3–29. DOI: 10.1016/S0021-9673(02)01336-5.
- [30] F. Chemat and M. A. Vian. Alternative solvents for natural products extraction. 381. Springer, 2014.
- [31] C. C. Teo, S. N. Tan, J.W. H. Yong, C. S. Hew, and E. S. Ong, (2010) “Pressurized hot water extraction (PHWE)" Journal of Chromatography A 1217(16): 2484–2494. DOI: 10.1016/j.chroma.2009.12.050.
- [32] M. Pillot, B. Lebeau, H. Nouali, T. J. Daou, J. Patarin, and A. Ryzhikov, (2019) “High pressure intrusion of water and LiCl aqueous solutions in hydrophobic KIT-6 mesoporous silica: Influence of the grafted group nature" Microporous and Mesoporous Materials 280: 248–255. DOI: 10.1016/j.micromeso.2019.02.006.
- [33] S. Cliffe, M. S. Fawer, G. Maier, K. Takata, and G. Ritter, (1994) “Enzyme assays for the phenolic content of natural juices" Journal of Agricultural and Food Chemistry 42(8): 1824–1828. DOI: 10.1021/jf00044a048.
- [34] M. S. Blois, (1958) “Antioxidant determinations by the use of a stable free radical" Nature 181(4617): 1199–1200. DOI: 10.1038/1811199a0.
- [35] S. Murugesan, D. Ravichandran, D. K. Lakshmanan, G. Ravichandran, V. Arumugam, K. Raju, K. Geetha, and S. Thilagar, (2020) “Evaluation of anti rheumatic activity of Piper betle L.(Betelvine) extract using in silicon, in vitro and in vivo approaches" Bioorganic Chemistry 103: 104227. DOI: 10.1016/j.bioorg.2020.104227.
- [36] D. C. Montgomery. Design and analysis of experiments, 8e Wiley E-text reg card. Nashville, TN: JohnWiley & Sons, 2013.
- [37] M. Naczk and F. Shahidi, (2004) “Extraction and analysis of phenolics in food" Journal of chromatography A 1054(1-2): 95–111. DOI: 10.1016/j.chroma.2004.08.059.
- [38] R. Vijayasankar and K. Ikhlas A, (2012) “An investigation of the vegetative anatomy of Piper sarmentosum, and a comparison with the anatomy of Piper betle (Piperaceae)" American Journal of Plant Sciences 2012:
- [39] Ü. Niinemets, (2018) “Storage of defense metabolites in the leaves of Myrtaceae: news of the eggs in different baskets" Tree physiology 38(10): 1445–1450. DOI: 10.1093/treephys/tpy115.
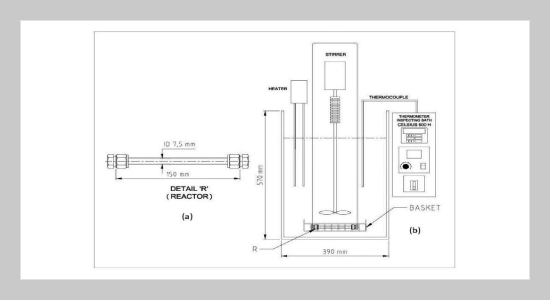
- [40] M. Khajenoori, A. H. Asl, and F. Hormozi, (2009) “Proposed models for subcritical water extraction of essential oils" Chinese Journal of Chemical Engineering 17(3): 359–365. DOI: 10.1016/S1004-9541(08)60217-7.