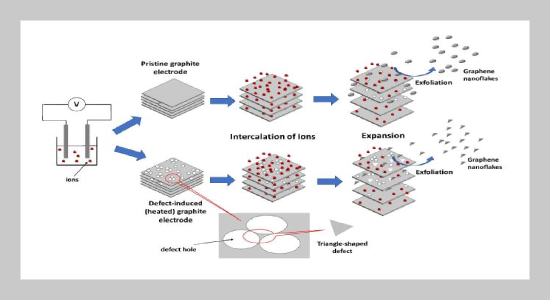
REFERENCES
- [1] Kong W., Kum H., Bae S. H., Shim J., Kim H., Kong L., Meng Y., Wang K., Kim, C. and Kim J., (2019) “Path towards graphene commercializationnm lab to market" Nature Nanotechnology 14(10): 927–938. DOI: https: //doi.org/10.1038/s41565-019-0555-2.
- [2] K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva, and A. A. Firsov, (2004) “Electric field in atomically thin carbon films" Science 306(5696): 666–669. DOI: 10 . 1126 / science.1102896.
- [3] S. Wei, R. Zhang, Y. Liu, H. Ding, and Y. L. Zhang, (2016) “Graphene quantum dots prepared from chemical exfoliation of multiwall carbon nanotubes: An efficient photocatalyst promoter" Catalysis Communications 74: 104–109. DOI: 10.1016/j.catcom.2015.11.010.
- [4] E. Kusrini, F. Oktavianto, A. Usman, D. P. Mawarni, and M. I. Alhamid, (2020) “Synthesis, characterization, and performance of graphene oxide and phosphorylated graphene oxide as additive in water-based drilling fluids" Applied Surface Science 506: 145005. DOI: 10.1016/ j.apsusc.2019.145005.
- [5] E. Kusrini, A. Suhrowati, A. Usman, M. Khalil, and V. Degirmenci, (2019) “Synthesis and characterization of graphite oxide, graphene oxide, and reduced graphene oxide from graphite waste using modified hummers’ method and zinc as reducing agent" International Journal of Technology 10(6): 1093–1104. DOI: 10.14716/ijtech. v10i6.3639.
- [6] F. Qing, Y. Hou, R. Stehle, and X. Li, (2019) “Chemical vapor deposition synthesis of graphene films" APL Materials 7(2): 020903. DOI: 10.1063/1.5078551.
- [7] S. H. Lee, D. Y. Kim, J. Lee, S. B. Lee, H. Han, Y. Y. Kim, S. C. Mun, S. H. Im, T. H. Kim, and O. O. Park, (2019) “Synthesis of Single-Crystalline Hexagonal Graphene Quantum Dots from Solution Chemistry" Nano Letters 19(8): 5437–5442. DOI: 10 . 1021 / acs . nanolett.9b01940.
- [8] W. H. Danial, A. Chutia, Z. A. Majid, R. Sahnoun, and M. Aziz. “Electrochemical synthesis and characterization of stable colloidal suspension of graphene using two-electrode cell system”. In: AIP Conference Proceedings. 1669. 2015, 020020. DOI: 10.1063/1.4919158.
- [9] W. H. Danial, N. A. Norhisham, A. F. Ahmad Noorden, Z. Abdul Majid, K. Matsumura, and A. Iqbal. A short review on electrochemical exfoliation of graphene and graphene quantum dots. 2021. DOI: 10.1007/s42823- 020-00212-3.
- [10] Y. L. Zhong and T. M. Swager, (2012) “Enhanced electrochemical expansion of graphite for in situ electrochemical functionalization" Journal of the American Chemical Society 134(43): 17896–17899. DOI: 10 . 1021 / ja309023f.
- [11] X. Wang and L. Zhang, (2019) “Green and facile production of high-quality graphene from graphite by the combination of hydroxyl radicals and electrical exfoliation in different electrolyte systems" RSC Advances 9(7):
3693–3703. DOI: 10.1039/c8ra09752f.
- [12] A. Ananthanarayanan, X. Wang, P. Routh, B. Sana, S. Lim, D. H. Kim, K. H. Lim, J. Li, and P. Chen, (2014) “Facile synthesis of graphene quantum dots from 3D graphene and their application for Fe3+ sensing" Advanced Functional Materials 24(20): 3021–3026. DOI: 10.1002/adfm.201303441.
- [13] Y. Yan, H. Li, Q. Wang, H. Mao, and W. Kun, (2017) “Controllable ionic liquid-assisted electrochemical exfoliation of carbon fibers for the green and large-scale preparation of functionalized graphene quantum dots endowed with multicolor emission and size tunability" Journal of Materials Chemistry C 5(24): 6092–6100. DOI: 10. 1039/c7tc01342f.
- [14] S. Yang, S. Brüller, Z. S. Wu, Z. Liu, K. Parvez, R. Dong, F. Richard, P. Samorì, X. Feng, and K. Müllen, (2015) “Organic Radical-Assisted Electrochemical Exfoliation for the Scalable Production of High-Quality Graphene" Journal of the American Chemical Society 137(43): 13927–13932. DOI: 10.1021/jacs.5b09000.
- [15] Y. Z. Htwe, W. S. Chow, Y. Suda, A. A. Thant, and M. Mariatti, (2019) “Effect of electrolytes and sonication times on the formation of graphene using an electrochemical exfoliation process" Applied Surface Science 469: 951–961. DOI: 10.1016/j.apsusc.2018.11.029.
- [16] Q. Zhou, G. Xia, M. Du, Y. Lu, and H. Xu, (2019) “Scotch-tape-like exfoliation effect of graphene quantum dots for efficient preparation of graphene nanosheets in water" Applied Surface Science 483: 52–59. DOI: 10. 1016/j.apsusc.2019.03.290.
- [17] S. Mutyala and J. Mathiyarasu, (2015) “Preparation of graphene nanoflakes and its application for detection of hydrazine" Sensors and Actuators, B: Chemical 210: 692–699. DOI: 10.1016/j.snb.2015.01.033.
- [18] C. Mansilla Wettstein, F. P. Bonafé, M. B. Oviedo, and C. G. Sánchez, (2016) “Optical properties of graphene nanoflakes: Shape matters" Journal of Chemical Physics 144(22): 224305. DOI: 10.1063/1.4953172. arXiv: 1512.00489.
- [19] S. Ahirwar, S. Mallick, and D. Bahadur, (2017) “Electrochemical Method to Prepare Graphene Quantum Dots and Graphene Oxide Quantum Dots" ACS Omega 2(11): 8343–8353. DOI: 10.1021/acsomega.7b01539.
- [20] M. J. Nine, T. T. Tung, and D. Losic. “Self-Assembly of Graphene Derivatives: Methods, Structures, and Applications”. In: Comprehensive Supramolecular Chemistry II. 9. Elsevier, 2017, 47–74. DOI: 10.1016/B978-0- 12-409547-2.12634-4.
- [21] Z. Zhao, P. Bai, W. Du, B. Liu, D. Pan, R. Das, C. Liu, and Z. Guo. An overview of graphene and its derivatives reinforced metal matrix composites: Preparation, properties and applications. 2020. DOI: 10.1016/j.carbon.2020. 08.040.
- [22] S. K. Tiwari, A. Huczko, R. Oraon, A. De Adhikari, and G. C. Nayak, (2017) “Facile electrochemical synthesis of few layered graphene from discharged battery electrode and its application for energy storage" Arabian Journal of Chemistry 10(4): 556–565. DOI: 10.1016/j. arabjc.2015.08.016.
- [23] I. Razado-Colambo, J. Avila, D. Vignaud, S. Godey, X. Wallart, D. P. Woodruff, and M. C. Asensio, (2018) “Structural determination of bilayer graphene on SiC(0001) using synchrotron radiation photoelectron diffraction" Scientific Reports 8(1): 10190. DOI: 10.1038/s41598- 018-28402-0.
- [24] M. Li and R. Luo. “Effect of heating rate on the properties of a graphite/phenolic-based composite”. In: Proceedings of the 5th International Conference on Information Engineering for Mechanics and Materials. 21. Paris: Atlantis Press, 2015. DOI: 10.2991/icimm-15. 2015.104.
- [25] S. Ruiz, J. A. Tamayo, J. D. Ospina, D. P. N. Porras, M. E. V. Zapata, J. H. M. Hernandez, C. H. Valencia, F. Zuluaga, and C. D. G. Tovar, (2019) “Antimicrobial films based on nanocomposites of chitosan/poly(Vinyl alcohol)/graphene oxide for biomedical applications" Biomolecules 9(3): 109. DOI: 10.3390/ biom9030109.
- [26] G. A. Ali, M. R. Thalji, W. C. Soh, H. Algarni, and K. F. Chong, (2020) “One-step electrochemical synthesis of MoS2/graphene composite for supercapacitor application" Journal of Solid State Electrochemistry 24(1): 25–34. DOI: 10.1007/s10008-019-04449-5.
- [27] B. Nazari, Z. Ranjbar, R. R. Hashjin, A. Rezvani Moghaddam, G. Momen, and B. Ranjbar, (2019) “Dispersing graphene in aqueous media: Investigating the effect of different surfactants" Colloids and Surfaces A: Physicochemical and Engineering Aspects 582: 123870. DOI: 10.1016/j.colsurfa.2019.123870.
- [28] S. Li, Y. Chen, X. He, X. Mao, Y. Zhou, J. Xu, and Y. Yang, (2019) “Modifying Reduced Graphene Oxide by Conducting Polymer Through a Hydrothermal Polymerization Method and its Application as Energy Storage Electrodes" Nanoscale Research Letters 14(1): 226. DOI: 10.1186/s11671-019-3051-6.
- [29] J. Peng, Z. Zhao, M. Zheng, B. Su, X. Chen, and X. Chen, (2020) “Electrochemical synthesis of phosphorus and sulfur co-doped graphene quantum dots as efficient electrochemiluminescent immunomarkers for monitoring okadaic acid" Sensors and Actuators, B: Chemical 304: 127383. DOI: 10.1016/j.snb.2019.127383.
- [30] R. V. Nair, R. T. Thomas, V. Sankar, H. Muhammad, M. Dong, and S. Pillai, (2017) “Rapid, Acid-Free Synthesis of High-Quality Graphene Quantum Dots for Aggregation Induced Sensing of Metal Ions and Bioimaging" ACS Omega 2(11): 8051–8061. DOI: 10 . 1021 / acsomega.7b01262.
- [31] F. Sharif, A. S. Zeraati, P. Ganjeh-Anzabi, N. Yasri, M. Perez-Page, S. M. Holmes, U. Sundararaj, M. Trifkovic, and E. P. Roberts, (2020) “Synthesis of a high-temperature stable electrochemically exfoliated graphene" Carbon 157: 681–692. DOI: 10.1016/j.carbon.2019.10. 042.
- [32] H. Huang, S. Yang, Q. Li, Y. Yang, G. Wang, X. You, B. Mao, H. Wang, Y. Ma, P. He, Z. Liu, G. Ding, and X. Xie, (2018) “Electrochemical Cutting in Weak Aqueous Electrolytes: The Strategy for Efficient and Controllable Preparation of Graphene Quantum Dots" Langmuir 34(1): 250–258. DOI: 10 . 1021 / acs . langmuir. 7b03425.
- [33] H. Kalita, V. S. Palaparthy, M. S. Baghini, and M. Aslam, (2020) “Electrochemical synthesis of graphene quantum dots from graphene oxide at room temperature and its soil moisture sensing properties" Carbon 165: 9– 17. DOI: 10.1016/j.carbon.2020.04.021.
- [34] Z. Zhao, W. Cai, Z. Xu, X. Mu, X. Ren, B. Zou, Z. Gui, and Y. Hu, (2020) “Multi-role p-styrene sulfonate assisted electrochemical preparation of functionalized graphene nanosheets for improving fire safety and mechanical property of polystyrene composites" Composites Part B: Engineering 181: 107544. DOI: 10.1016/j. compositesb.2019.107544.