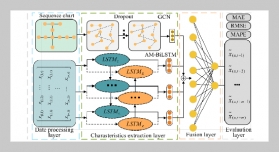
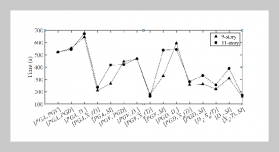
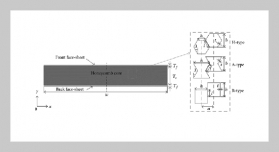
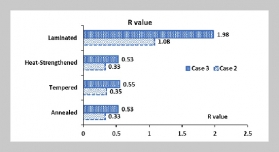
REFERENCES
- [1] A. Kalra and A. Gupta, (2020) “Recent advances in decolourization of dyes using iron nanoparticles: A mini review" Materials Today: Proceedings: DOI: 10.1016/j.matpr.2020.04.677.
- [2] Y. A¸sçi, (2013) “Decolorization of Direct Orange 26 by heterogeneous Fenton oxidation" Desalination andWater Treatment 51(40-42): 7612–7620. DOI: 10.1080/19443994.2013.776504.
- [3] M. E. M. Ali, T. A. Gad-Allah, and M. I. Badawy, (2013) “Heterogeneous Fenton process using steel industry wastes for methyl orange degradation" AppliedWater Science 3(1): 263–270. DOI: 10.1007/s13201-013-0078-1.
- [4] P. A. Carneiro, R. F. Nogueira, and M. V. B. Zanoni, (2007) “Homogeneous photodegradation of C.I. Reactive Blue 4 using a photo-Fenton process under artificial and solar irradiation" Dyes and Pigments 74(1): 127–132.DOI: 10.1016/j.dyepig.2006.01.022.
- [5] N. N. Nassar, N. N. Marei, G. Vitale, and L. A. Arar, (2015) “Adsorptive removal of dyes from synthetic and real textile wastewater using magnetic iron oxide nanoparticles: Thermodynamic and mechanistic insights" The Canadian Journal of Chemical Engineering 93(11):1965–1974. DOI: 10.1002/cjce.22315.
- [6] J. J. Pignatello, E. Oliveros, and A. MacKay, (2006) “Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry" Critical Reviews in Environmental Science and Technology 36(1): 1–84. DOI: 10 . 1080 /10643380500326564.
- [7] E. Neyens and J. Baeyens, (2003) “A review of classic Fenton’s peroxidation as an advanced oxidation technique" Journal of Hazardous Materials 98(1-3): 33–50.
- [8] A. Babuponnusami and K. Muthukumar, (2014) “A review on Fenton and improvements to the Fenton process for wastewater treatment" Journal of Environmental Chemical Engineering 2(1): 557–572. DOI: 10.1016/j.jece.2013.10.011.
- [9] Y. Zhu, R. Zhu, Y. Xi, J. Zhu, G. Zhu, and H. He, (2019) “Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review" Applied Catalysis B: Environmental 255: 117739. DOI: 10.1016/j.apcatb.2019.05.041.
- [10] P. V. Nidheesh, (2015) “Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: a review" RSC Advances 5(51): 40552–40577.DOI: 10.1039/C5RA02023A.
- [11] S. Yoon and S. Bae, (2019) “Novel synthesis of nanoscale zerovalent iron from coal fly ash and its application in oxidative degradation of methyl orange by Fenton reaction" Journal of Hazardous Materials 365: 751–758. DOI:10.1016/j.jhazmat.2018.11.073.
- [12] A. J. dos Santos, G. da Costa Cunha, D. R. S. Cruz, L. P. C. Romão, and C. A. Martínez-Huitle, (2019) “Iron mining wastes collected from Mariana disaster: Reuse and application as catalyst in a heterogeneous electro-Fenton process" Journal of Electroanalytical Chemistry 848: 113330. DOI: 10.1016/j.jelechem.2019.113330.
- [13] H. T. Van, L. H. Nguyen, T. K. Hoang, T. P. Tran, A. T. Vo, T. Pham, and X. Nguyen, (2019) “Using FeOconstituted iron slag wastes as heterogeneous catalyst for Fenton and ozonation processes to degrade Reactive Red 24 from aqueous solution" Separation and Purification Technology 224: 431–442. DOI: 10.1016/j.seppur.2019.05.048.
- [14] USEPA. Sustainable Management of Construction and Demolition Materials. 2018.
- [15] M. Osmani. “ConstructionWaste”. In:Waste. Elsevier, 2011, 207–218. DOI: 10 . 1016/B978 - 0 - 12 - 381475 -3.10015-4.
- [16] N. H. Hoang, T. Ishigaki, R. Kubota, T. K. Tong, T. T. Nguyen, H. G. Nguyen, M. Yamada, and K. Kawamoto, (2020) “Waste generation, composition, and handling in building-related construction and demolition in Hanoi, Vietnam" Waste Management 117: 32–41.DOI: 10.1016/j.wasman.2020.08.006.
- [17] S. M. El-Haggar. “Sustainability of Construction and Demolition Waste Management”. In: Sustainable Industrial Design and Waste Management. Elsevier, 2007,261–292. DOI: 10.1016/B978-012373623-9/50010-1.
- [18] A. Akhtar and A. K. Sarmah, (2018) “Construction and demolition waste generation and properties of recycled aggregate concrete: A global perspective" Journal of Cleaner Production 186: 262–281. DOI: 10.1016/j.jclepro.2018.03.085.
- [19] R. Janani and V. Kaveri, (2020) “A critical literature review on reuse and recycling of construction waste in construction industry" Materials Today: Proceedings:
- [20] N. A. Youssef, S. A. Shaban, F. A. Ibrahim, and A. S. Mahmoud, (2016) “Degradation of methyl orange using Fenton catalytic reaction" Egyptian Journal of Petroleum 25(3): 317–321.
- [21] S. Beldjoudi, K. Kouachi, S. Bourouina-Bacha, G. Lafaye, and A. Soualah, (2020) “Kinetic study of methyl orange decolorization by the Fenton process based on fractional factorial design" Reaction Kinetics, Mechanisms and Catalysis 130(2): 1123–1140.
- [22] H.-Y. Xu, T.-N. Shi, L.-C. Wu, and S.-Y. Qi, (2013) “Discoloration of methyl orange in the presence of school and H 2 O 2: Kinetics and mechanism" Water, Air, & Soil Pollution 224(10): 1–11.
- [23] Y. Wang, Y. Gao, L. Chen, and H. Zhang, (2015) “Goethite as an efficient heterogeneous Fenton catalyst for the degradation of methyl orange" Catalysis Today 252:107–112. DOI: 10.1016/j.cattod.2015.01.012.
- [24] N. Panda, H. Sahoo, and S. Mohapatra, (2011) “Decolourization of Methyl Orange using Fenton-like mesoporous Fe2O3–SiO2 composite" Journal of Hazardous Materials 185(1): 359–365. DOI: 10.1016/j.jhazmat.2010.09.042.
- [25] Y. Liu, G. Zhang, S. Chong, N. Zhang, H. Chang, T. Huang, and S. Fang, (2017) “NiFe(C2O4)x as a heterogeneous Fenton catalyst for removal of methyl orange" Journal of Environmental Management 192: 150–155. DOI: 10.1016/j.jenvman.2017.01.064.
- [26] G.-Q. Huang, G.-J. Qi, T.-Y. Gao, J. Zhang, and Y.-H. Zhao, (2020) “Fe-pillared montmorillonite as effective heterogeneous Fenton catalyst for the decolorization of methyl orange" Journal of Chemical Sciences 132(1):116. DOI: 10.1007/s12039-020-01820-2.
- [27] L.Wang, J. Yang, Y. Li, J. Lv, and J. Zou, (2016) “Removal of chlorpheniramine in a nanoscale zero-valent iron induced heterogeneous Fenton system: Influencing factors and degradation intermediates" Chemical Engineering Journal 284: 1058–1067. DOI: 10.1016/j.cej.2015.09.042.
- [28] Y. Yuan, B. Lai, and Y.-Y. Tang, (2016) “Combined Fe0/air and Fenton process for the treatment of dinitrodiazophenol (DDNP) industry wastewater" Chemical Engineering Journal 283: 1514–1521. DOI: 10.1016/j.cej.2015.08.104.
- [29] T. Zhou, Y. Li, J. Ji, F.-S. Wong, and X. Lu, (2008) “Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: Kinetic, pathway and effect factors" Separation and Purification Technology 62(3): 551–558. DOI: 10.1016/j.seppur.2008.03.008.
- [30] L. Xu and J. Wang, (2011) “A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol" Journal of hazardous materials 186(1): 256–264.
- [31] S. Zha, Y. Cheng, Y. Gao, Z. Chen, M. Megharaj, and R. Naidu, (2014) “Nanoscale zero-valent iron as a catalyst for heterogeneous Fenton oxidation of amoxicillin" Chemical Engineering Journal 255: 141–148.DOI: 10.1016/j.cej.2014.06.057.
- [32] F. Velichkova, C. Julcour-Lebigue, B. Koumanova, and H. Delmas, (2013) “Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano) particles" Journal of Environmental Chemical Engineering 1(4):1214–1222.
- [33] S. Adityosulindro, C. Julcour, and L. Barthe, (2018) “Heterogeneous Fenton oxidation using Fe-ZSM5 catalyst for removal of ibuprofen in wastewater" Journal of Environmental Chemical Engineering 6(5): 5920–5928.DOI: 10.1016/j.jece.2018.09.007.
- [34] L. Gomathi Devi, S. Girish Kumar, K. Mohan Reddy, and C. Munikrishnappa, (2009) “Photo degradation of methyl orange an azo dye by advanced Fenton process using zero valent metallic iron: influence of various reaction parameters and its degradation mechanism." Journal of hazardous materials 164(2-3): 459–67. DOI: 10.1016/j.jhazmat.2008.08.017.
- [35] S. Lin and M. D. Gurol, (1998) “Catalytic Decomposition of Hydrogen Peroxide on Iron Oxide: Kinetics, Mechanism, and Implications" Environmental Science & Technology 32(10): 1417–1423. DOI: 10.1021/es970648k.
- [36] A. L.-T. Pham, C. Lee, F. M. Doyle, and D. L. Sedlak, (2009) “A Silica-Supported Iron Oxide Catalyst Capable of Activating Hydrogen Peroxide at Neutral pH Values" Environmental Science and Technology 43(23):8930–8935. DOI: 10.1021/es902296k.
- [37] X. Zeng and A. T. Lemley, (2009) “Fenton Degradation of 4,6-Dinitro-o-cresol with Fe2+-Substituted Ion-Exchange Resin" Journal of Agricultural and Food Chemistry 57(9): 3689–3694. DOI: 10.1021/jf900764q.
- [38] J. He, X. Yang, B. Men, and D. Wang, (2016) “Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review" Journal of Environmental Sciences: DOI: 10.1016/j.jes.2015.12.003.
- [39] I.-H. Yoon, G. Yoo, H.-J. Hong, J. Kim, M. G. Kim, W.-K. Choi, and J.-W. Yang, (2016) “Kinetic study for phenol degradation by ZVI-assisted Fenton reaction and related iron corrosion investigated by X-ray absorption spectroscopy" Chemosphere 145: 409–415. DOI: 10.1016/j.chemosphere.2015.11.108.